NEONATOLOGY ON THE WEB
Historical Review and Recent Advances
in Neonatal and Perinatal Medicine
Edited by George F. Smith, MD and Dharmapuri Vidyasagar, MD
Published by Mead Johnson Nutritional Division, 1980
Not Copyrighted By Publisher
Chapter 10
The Obstetric View of Premature Labor
M. Yusoff Dawood, M. D., Ch. B.
Mcduff was from his mother's womb.
Untimely ripped
-- Shakespeare: Macbeth V. vii
DEFINITION
According to the World Health Organization (WHO)
recommendation,[1] preterm labor is defined
as labor starting earlier than 37 completed weeks (less than 259
days) from the first day of the last menstrual period and the term
premature should no longer be used. The term premature
was previously defined as a birth weight of less than 2500 gm.
Therefore, this will include infants of low birth weight due to early
delivery or due to growth retardation but exclude infants of preterm
labor that have gestational weights of more than 2500 gm such as in
diabetes mellitus. The problems of the preterm infant are closely
associated with chronological maturity rather than birth weight per
se and it is, therefore, more meaningful to adopt the WHO
nomenclature of preterm labor. Nevertheless, adoption of the term
preterm labor is not without practical problems in pregnancies
where the date of the last menstrual period is in doubt or unknown.
Infants weighing less than 2500 gm are, therefore, designated as
low birth weight (LBW).
INCIDENCE
The true incidence of preterm births varies from country to
country and from one geographic region to another within a country.
In 1969, the incidence of preterm births in the United States was
9.8% of all pregnancies.[2] In Britain, 6.4% of
all births were preterm in 1970, compared to 3.4% of all births in
East Germany in 1974.[3] Nevertheless, preterm
births account for 75-85% of early neonatal deaths which are not due
to lethal congenital malformations.[4] Thus
preterm births represent a sizeable proportion of potentially
preventable causes of perinatal mortality and morbidity in developed
countries.
ETIOLOGY
Epidemiologic Factors. A number of epidemiologic factors
have been implicated as being associated with an increased frequency
of preterm labor but such associations do not necessarily indicate a
direct cause and effect relationship. Further, in many of the
studies, there was no cut distinction between preterm and
term growth retarded infants, since the definition used was
premature infants (based on birth weight) or low birth weight
infants. Factors that have been associated with preterm labor, but
where no cause and effect relationship can be clearly demonstrated,
include race,[2,5] ages social
environment,[6] socio-economic
status,[7] marital status,[8,9]
use of narcotics[10] and previous pregnancy
performance such as parity, previous abortion and preterm labor,
previous fetal and neonatal deaths and previous bleeding and
isoimmunization. Black women have a higher incidence of preterm birth
(18.6%) than white women (8.3%).[2] In the
Obstetrical Statistical Cooperative study from 1970 through 1976,
non-whites had 7% of preterm low birth weight babies compared with
3.4% in whites.[5] Women of all racial groups
under 15 years of age had the highest incidence of preterm low birth
weight infants while women aged 25-29 years had the lowest incidences
Boldman and Reeds noted that the incidence of low birth weight
infants was increased in urban living, low per capita income, low per
capita energy consumption, low newspaper circulation, lack of radio
and television and low density of physicians. The "ward" (pavilion)
patients had a 67-84% increase in incidence of preterm births
compared with the "private" patients.[5]
Baird[7] found that the wives of laborers had the
highest incidence of low birth weight babies, with the lowest
incidence in wives of farmers and wives of craftsmen and
professionals somewhere in between. Unmarried whites, but not
non-whites, had a 90% higher incidence of preterm births than married
whites.[5] However, illegitimate pregnancies had a
higher incidence of low birth weight babies (term and preterm) for
all racial groups.[8,9] Smoking increases the
risks for both preterm births and lower gestational
age.[11,12] Likewise, the use of narcotics also
increases preterm births. Low birth weights (both term and preterm)
were most common among the first[9,13] and after
the fourth pregnancy.[9,14] However, parity does
not seem to have a significant effect on the incidence of preterm
births per se.[5] A history of previous abortion,
preterm labor, fetal and neonatal deaths, antepartum bleeding
(placenta praevia and abruptio placentae and isoimmunization are all
associated with an increased incidence of preterm
births).[5,10] The risks of preterm labor with
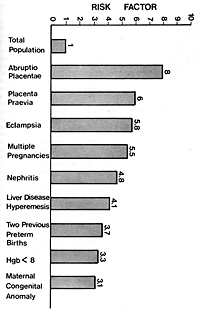
some of these associated factors are summarized in Figure 1.
Etiologic Factors. The etiologic factors that have been
frequently implicated in preterm labor can be classified into four
major categories: maternal, fetoplacental, uterine and iatrogenic
which are listed in Table 1. The majority of cases of preterm labor
are without apparent or detectable cause and are often referred to as
idiopathic preterm labor. A definitely avoidable iatrogenic cause of
preterm labor is elective delivery prior to term because of an error
in estimated gestational age. Greater reliance on fetal
ultrasonographic cephalometry and tests of fetal pulmonary maturity
have greatly improved accuracy of estimating gestational age but have
not totally eliminated the iatrogenic cause of preterm births. Acute
systemic illness in the mother with pyrexia, such as in
pyelonephritis and febrile viral infections, can cause preterm labor
and the increased uterine activity has been attributed to the
prostaglandins released during pyrexia. Severe chronic systemic
illnesses, such as chronic renal and cardiac diseases or chronic
hypertension, are known to be associated with low birth weight
infants but not necessarily preterm labor. Maternal endocrine causes
giving rise to preterm labor include
hyperthyroidism,[15]
hyperparathyroidism[16] and
hyperadrenocorticism.[17] Trauma and injuries to
the abdomen and gravid uterus can obviously induce preterm labor.
Several studies have shown that women experiencing more orgasm with
coitus in late pregnancy have in creased uterine activity and an
increased incidence of preterm labor.[18,20]
Coital activity appears to be significantly greater in women having
preterm labor than in women with term labor, only when there is no
obvious reason for the preterm labor.[21,22] The
incidence of preterm labor and delivery is increased in women who
have previously had preterm births or delivery of low birth weight
infants .23 Certain genital pathogens such as mycoplasmas
are isolated more frequently from the cervices of patients with a
history of preterm labor .24 Nevertheless, the
relationship between vaginal infections and preterm labor has not
been unequivocally established.
Genetic abnormalities of the fetus are associated with preterm
labor and fetal death in utero usually results in delivery of
the fetus within two to three weeks. Antepartum hemorrhage, from
placenta praevia or abruptio placenta, is associated
with an increased incidence of preterm
labor.[23,25]
Uterine factors such as overdistension of the uterus from
polyhydramnios or multiple pregnancies often produce increased
uterine activity and result in preterm labor. Preterm labor occurs in
30-47% of twin gestation and is twice as common in women with
polyhydramnios compared to those with uncomplicated
pregnancy.[26] Ten percent of all preterm
deliveries are accounted for by multiple
pregnancies.[5] The presence of an intrauterine
contraceptive device increases the risk of preterm
labor.[27] In women with congenital malformation
of the uterus, the incidence of preterm labor may be as high as 20%,
if the pregnancy continues beyond 20 weeks' gestation. More recently,
16% of patients who had spontaneous preterm labor, without any
obvious complication of pregnancy, were found to have congenital
fundal abnormalities of the uterus and an additional 8% had
incompetent cervix.[28] These were
ultrasonographically demonstrable in the puerperium compared to women
delivering at term who had no such demonstrable anomalies. Premature
rupture of the membranes precedes 15 to 34% of cases of preterm
labor,[5,29,30] but the crux of the problem lies
in the cause of the premature rupture of the membranes as the factor
responsible for initiating the premature uterine activity rather than
the rupture of the membrane itself. Indeed there is some preliminary
support for the contention that intraamniotic infection may initiate
preterm labor without premature rupture of the
membranes.[31] Mechanical dilatation of the cervix
to greater than 8 mm has been held responsible for the increased risk
of preterm births seen in women who had previous induced
abortions.[3,32-34] With the recent enthusiasm and
recommendation for dilatation and evacuation for induced abortion up
to 14 to 16 weeks' gestation, cervical trauma and incompetence may
constitute a significant factor accounting for preterm labor in the
future.
Iatrogenic causes of preterm labor are by far frequently due to
induction of labor where the duration of pregnancy can be seriously
questioned or where there is an urgent need for terminating the
pregnancy due to maternal or fetal interests. Certainly the threefold
increase of preterm labor associated with Rh isoimmunization is
partially iatrogenic.[5]
Table 1
Etiologic Factors in Preterm Labor
|
A. Maternal
|
B. Uterine
|
C. Fetoplacental
|
D. Iatrogenic
|
E. Idiopathic
|
|
1. Systemic Illness
|
l. Overdistension
|
1. Genetic Abnormalities
|
1. Wrong Dates
|
|
|
2. Endocrine Disorders
|
2. Malformation
|
2. Fetal Death
|
2. Fetal or Maternal Indication
|
|
|
3. Trauma
|
3. Infection
|
3. Abruptio Placenta
|
|
|
|
4. Orgasm
|
4. Foreign Body
|
4. Placenta Praevia
|
|
|
|
5. Genital Infection
|
5. Rupture of Membranes
|
|
|
|
|
|
6. Cervical Trauma
|
|
|
|
MECHANISM OF ONSET OF LABOR
The exact hormonal mechanisms regulating the onset and maintenance
of human labor at term are not understood and that of preterm labor
is even less so. Many of the hormonal mechanisms applied to humans
are extrapolated from animal studies[35] and it is
now clear that such data are not applicable to the parturient woman.
Many endocrine changes have been demonstrated in women near or at the
time of labor but consistently similar changes have not yet been
demonstrated in idiopathic preterm labor. Again, the sequence of such
hormonal changes has not been conclusively demonstrated in human
labor at term. The mechanism of action of hormones on the uterine
muscle is dependent on the availability of their receptors in the
myometrium. The final mediator of myometrial contraction is at the
level of intracellular free ionic calcium (Ca++) (see below). Changes
in hormone level will affect myometrial function through their own
receptors or induction of other hormone receptors; and, through
alteration of collagen or connective tissue turnover, to render the
myometrium more distensible and elastic.
Steroid Hormones. The overall uterine effect of estrogen is
to increase contractility and responsiveness to other uterotonic
stimuli. Circulating estrogen levels increase throughout pregnancy.
It is now generally agreed in most studies that maternal estrogen
levels increase in the last few weeks prior to parturition. Estrogens
increase production of uterine prostaglandins which are an important
uterine stimulant. Additionally, in subprimate species, estrogens
have also been demonstrated to induce and increase myometrial
oxytocin receptors.[36,37] In rabbits, estrogens
increase the number of alpha-adrenergic receptors which mediate
contraction.[38] Thus estrogens can enhance
uterine activity via one or more of all these three mechanisms. On
the other hand, the overall effect of progesterone has been
associated with uterine quiescence. Progesterone has been shown to
decrease uterine oxytocin receptors[36] and
increase beta-adrenergic receptors, which are associated with uterine
relaxation in animals.[38] Progesterone has also
been proposed to inhibit locally the progressive increases in the
active portion of the uterine wall responding to uterotonic stimuli
and is responsible for the poorer elasticity of the
uterus.[39,40] Except for one study showing a
significant fall in maternal circulating progesterone in the last few
weeks prior to spontaneous term labor, the majority of reports have
been unable to demonstrate the decline.[41]
Likewise, changes in the estrogen/progesterone ratio which may
indirectly reflect the opposing effects of these two hormones on
uterine activity could not be demonstrated both in term and preterm
labor.[42] Nevertheless, a local fall in uterine
progesterone may still be operative but has not yet been demonstrated
in women. However, there is an increase in progesterone-binding
protein in the fetal membranes in the last few weeks of pregnancy,
with a decrease in the metabolism of progesterone by these membranes,
thus suggesting a local intracellular fall of
progesterone.[43,44] This leads to a labilization
of lysosomes and release of the enzyme phospholipase A2 which
promotes the generation of arachidonic acid, an obligatory precursor
and the rate limiting factor in the biosynthesis of prostaglandins.
Changes in both maternal peripheral plasma estrogen and progesterone
levels are, therefore, at best, inconclusive and contradictory. While
elevated plasma estradiol and low progesterone levels have been found
by some,[44,45] others have found no change in the
estrogen/progesterone ratio in women with idiopathic preterm
labor.[42,46] There are no published studies
examining steroid hormone levels prior to the onset of preterm labor.
Although in many animal species, release of corticosteroids
appears to be an important endocrine mechanism involved in the onset
of parturition, there is insufficient evidence of such a role in
human labor. Earlier suggestions of increased maternal or fetal
cortisol levels in women undergoing spontaneous term labor have not
been subsequently supported.
Prostaglandins. Both prostaglandins E2 and F2a stimulate
myometrium from pregnant human uterus. There is a substantial body of
evidence to indicate that prostaglandins are present during
spontaneous term labor and play an important role in uterine
contractions during human labor. However, it is still unclear whether
prostaglandin is responsible for initiating the labor or is merely an
intermediate in the much more complex endocrine hierarchical changes
that must occur in order to trigger labor. Increased levels of
prostaglandins and their metabolites have been found in amniotic
fluid and peripheral blood in spontaneous
labor.[47-50] However, the evidence for
prostaglandins triggering idiopathic preterm labor is much less
persuasive. While several studies are able to demonstrate increased
prostaglandins or their metabolite in the maternal blood or amniotic
fluid when preterm labor is progressive, none has shown such changes
prior to the onset of preterm labor or even in those that are preterm
labor which ceased to progress.[51] Nonetheless,
preliminary studies on the use of prostaglandin inhibitors in
arresting human preterm labor indicate a measurable degree of success
with some of these agents.
Oxytocin. Oxytocin has been well established as an agent
for stimulating uterine contractions and induction of labor. Oxytocin
receptors have been demonstrated in human myometrium and mammary
tissue[37,52] and, in animals, estrogens increase
while progesterone decreases the uterine oxytocin receptors. There is
now incontrovertible evidence for the presence of oxytocin in both
the maternal and the fetal circulation, with the fetal contribution
being more evident in the first stage and the maternal contribution
more obvious in the second stage of labor.[53-56]
While it is not clear if oxytocin levels are altered in preterm
labor, alcohol appears to suppress the episodic release of oxytocin
in term labor.[57] The efficacy of ethanol in the
treatment of preterm labor has been credited to this mechanism of
action which is more readily demonstrable in the
rabbit.[58] The role of oxytocin in human
parturition does have a novel appeal. Because the action of oxytocin
is limited to a few tissues (breast and uterus), oxytocin analogues
which antagonize uterine oxytocin receptors, with little or no side
effects, could be developed. Indeed, there is some preliminary hope
for such an antagonist which is being evaluated in laboratory
animals.[59]
It is increasingly obvious that clinical observations suggest that
the mechanism of onset of term and preterm labor involves the removal
of one or more of the complex multiple systems involved in
maintaining uterine quiescence and preservation of the pregnancy. The
type of mechanism involved may vary from situation to situation, even
in spontaneous term labor, and may thus account for the failure to
explain satisfactorily and conclusively any one mechanism in all
instances. Indeed this wide latitude and ability to recruit more than
one mechanism to initiate labor, depending on the circumstances, may
confer an advantage in the economy of human reproduction and
parturition. Nevertheless, that intracellular ionic alteration,
namely free ionic calcium, is the final modulator of myometrial
activity can be readily accepted. It is also the final mechanism by
which all modalities of arresting preterm labor ultimately work
directly or indirectly. Thus it is obvious that no single method of
treating preterm labor is necessarily going to be of therapeutic
benefit to all women with preterm labor.
Intracellular Regulation of Myometrial Contraction. Myosin
is the principal protein of muscle contraction. It is an enzyme that
converts the chemical energy of ATP to mechanical movement during the
contraction process. The types of myosins found during pregnancy are
different from those during nonpregnancy and such changes in the
myometrial myosin appear to be specific to pregnancy and
labor.[60]
Calcium appears to play a central role in the regulation of muscle
contractility. Calcium is stored in the sarcoplasmic reticulum and
accumulation of calcium in the sarcoplasmic reticulum is an
ATP-dependent enzymatic process.
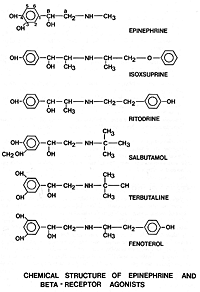
In the myometrial cell, the presence of calcium promotes
actin-myosin interaction (Figure 2). Myosin must be phosphorylated
before it can interact with actin.[61]
Phosphorylation of myosin is catalyzed by the activity of myosin
light-chain kinase, an enzyme that is activated by calcium.
Phosphorylated actomyosin causes myometrial contraction. Myometrial
relaxation occurs when the myosin is dephosphorylated by the enzyme
myosin light-chain phosphatase. Actin does not recognize
dephosphorylated myosin and, therefore, no interaction occurs. Thus
the contractile state of the smooth muscle is the result of the
relative activity of the kinase and phosphatase enzymes.
Myosin light-chain kinase is, therefore, the key regulator of
smooth muscle contractility. This enzyme is modulated by three
cellular regulatory systems, namely:
1. Calcium (10-6 - 10-7
M) is necessary for myosin light-chain kinase activity,
2. Calmodulin (calcium dependent regulatory protein) must be
associated with myosin light-chain kinase activity to activate the
enzyme,[62] and
3. Phosphorylation of the myosin light-chain kinase by cAMP
dependent protein kinase inhibits myosin light-chain kinase
activity.[63]
These three regulatory mechanisms are affected by hormones and
pharmacologic agents. Prostaglandin F2a, oxytocin and acetylcholine
raise intracellular calcium and, therefore, promote uterine
contractility. Progesterone, cAMP and isoproterenol promote calcium
uptake and, therefore, depress uterine contractility.
PREDICTION OF POTENTIAL PRETERM LABOR
The high-risk factors that are associated with preterm birth have
been used by several investigators to predict preterm labor and
identify such patients for special management. Such attempts have so
far met with poor accuracy.[64-67] Using high-risk
factors such as age, weight, social class, smoking, threatened or
previous abortion and previous poor history,
Fedrick[66] correctly identified only 9% of
primigravida and 25% of multigravida with preterm births. In another
study, preterm births were predicted three times more than what
eventually occurred,[67] although the correctly
predicted preterm births accounted for two thirds of all preterm
births. Unless such predictive attempts are used only for passive
management (such as restricting physical activities), active
treatment (such as with prophylactic oral tocolytic agents) may
unnecessarily expose those who will not go into preterm labor to
unneeded treatment that is not totally innocuous. Nevertheless, such
identification will put those patients that are likely to go into
preterm labor into a high risk category, thus warranting closer
prenatal attention.
DIAGNOSIS
When preterm labor cannot be prevented, early diagnosis is the key
to successful arrest of preterm labor. However, early diagnosis is
often difficult until progressive cervical dilatation has occurred.
Consequently, many patients receive treatment after preterm labor has
become established or when such labor has progressed to an
irreversible stage. On the other hand, many patients who may not be
in preterm labor may unwittingly receive potent tocolytic agents and
are, therefore, unnecessarily exposed to their side effects. Hence,
it is crucial that the first step is to differentiate true
from false labor, preterm from term gestation and
complicated from uncomplicated pregnancies. If preterm
labor is diagnosed, it becomes necessary to use sound judgment to
assess the relative risk of preterm parturition compared to continued
pregnancy. Optimum management, therefore, requires an accurate
diagnosis.
Spontaneous uterine contractions occur and increase throughout
gestation. Such spontaneous uterine contractions are of two types,
the regular, high-frequency and low-intensity contractions which are
usually imperceptible, and the irregular, low-frequency and
high-intensity (10-15 mm Hg), Braxton-Hicks contractions. The uterine
contractions of established or progressive labor are characterized by
increase in frequency, intensity and regularity. False
labor can be distinguished from true labor by the irregular,
infrequent, painless and low-intensity features of the uterine
contractions in the former. Nevertheless, it is sometimes difficult
to distinguish false from true labor, since the contractions of false
labor can be painful and the uterine contractions during active labor
can be irregular.[68,69] Cervical dilatation is a
better distinguishing feature since dilatation occurs with
established progressive true labor in response to the high-intensity,
high-frequency contraction. However, there is no conclusive evidence
that any significant degree of early cervical effacement and
dilatation accurately differentiates active preterm labor. In more
than 50% of pregnant women, the internal cervical os is dilated one
finger breadth or more by 31 to 36 weeks'
gestation.[70,71] It should be emphasized that
early cervical dilatation is more commonly found in multiparas, one
third of whom may have a cervical dilatation of two to three
centimeters by late midtrimester.[72,73] Based on
the study of cervical dilatation during the last four weeks prior to
term, a cervical dilatation of up to 2.1 centimeters is within two
standard deviations of the mean, four weeks prior to term
labor.[74] Nevertheless, a couple of studies have
indicated that the demonstration of frequent regular contractions
(three or more contractions over a 10 minute period), cervical
effacement and early cervical dilatation, not exceeding 3 to 4
centimeters, were associated with preterm
births.[75,76] Using such criteria, early
prediction of labor would include only 20 to 29% of cases of false
labor. Therefore, to make a diagnosis of preterm labor the following
criteria should he made:
1. Regular painful contractions less than 10 minutes
apart and lasting at least 30 seconds. These contractions
should be present for at least 60 minutes.
2. Primigravid patients with intact membranes should have at
least 3 centimeters of cervical dilatation in the presence
of uneffaced cervix.
3. Multiparous patients with intact membranes in whom
the cervix is uneffaced should have at least four centimeters of
cervical dilatation.
4. Any patient with cervical effacement of 75% or more or with
progressive cervical dilatation or effacement.
One difficulty in deciding whether labor is preterm or otherwise,
is the inability of patients to remember their last menstrual period
and, therefore, the correct gestational age. While a fetal size of
2000 gm. or more, in the absence of any other obstetric complication,
is associated with the lower morbidity and mortality, the clinical
assessment of fetal weight may differ from the true weight by a
significant degree (200 gm. or more) when the fetal weight is in the
lower range. Thus, in patients who have irregular cycles or have
forgotten their last menstrual period, ultrasonographic cephalometry
becomes necessary-and also when there is apparent disparity between
the gestational age and the estimated fetal weight. The traditional
method for evaluation of gestational age includes the date of onset
of audible fetal heart tones, the date of onset of quickening, the
date of the last menstrual period, regularity of the menstrual cycle
and clinical assessment of uterine size and fundal height. Patients
who are unregistered or who have questionable gestational age will
require other methods to determine the true gestational age.
Ultrasonographic cephalometry can be used to determine fetal
biparietal diameter and gestational age, but the accuracy for
gestational dating is better if performed during the midtrimester.
Measurements of fetal abdominal circumference, ratio of head to trunk
circumferences and total intrauterine volume provide additional
indices of fetal weight and fetal growth. In addition, amniocentesis
may be indicated to assess fetal maturity by performing the
lecithin-sphingomyelin ratio, the foam test, or examining the
creatinine concentration and percentage of lipid positive cells in
the amniotic fluid. Between 32 and 36 weeks, amniocentesis
should be considered prior to inhibition of labor, since a
lecithinsphingomyelin ratio indicative of fetal pulmonary maturity is
evident in 66% of pregnancies and may mitigate against the use
of labor inhibiting drugs. While only 13% of such infants of
this gestational age have developed respiratory distress
syndrome,[77] pulmonary maturity in the fetus does
not assure maturity of other organ systems, and other problems
associated with prematurity may still occur. Thus, the decision not
to treat a woman in preterm labor should not be made only on the
basis of her mature lecithin-sphingomyelin ratio.
MANAGEMENT OF PRETERM LABOR
The management of preterm labor can be approached from three
different but non-exclusive aspects, namely:
1. Prevention of Preterm Labor
2. Inhibition and arrest of Preterm Labor
3. Improved obstetric and neonatal management of preterm
infant.
Prevention of Preterm Labor. Prevention of preterm labor is
probably the most effective and least expensive way of reducing
preterm labor and the associated perinatal morbidity and mortality.
Prevention, therefore, focuses on high-risk factors that are
associated with preterm labor. These high-risk factors have been
described earlier (vide supra). Appropriate family planning
and counselling should reduce unwanted pregnancies. Educational and
social reforms and nutritional counselling are required for low
socioeconomic factors. Elimination of smoking and excessive alcohol
consumption is necessary when these factors contribute to preterm
labor. Early diagnosis and appropriate treatment are required for
hypertension, diabetes mellitus, anemia, hyperthyroidism and urinary
tract infection. Early diagnosis and bedrest are indicated in
multiple pregnancies and in patients with cardiac disease,
cardiovascular disorders, placenta previa, and polyhydramnios.
Maternal infections require early treatment with antibiotics. Those
with repeated abortions or preterm labor due to congenital
malformation of the uterus require surgical correction of the
anomaly. Repetitive idiopathic preterm labor and those having
high-risk factors for preterm labor should probably refrain from
sexual intercourse in the third trimester or at least refrain from
vigorous sexual intercourse that may give rise to excessive orgasm
with risk of premature uterine activity.
Uterine tocolysis has been recommended in some centers whenever a
procedure is performed that could provide premature uterine activity.
For example, prophylactic ethanol therapy (vide infra) has
been recommended whenever a cervical cerclage, appendectomy or other
pelvic surgical procedure is performed during pregnancy. More
recently 17a-hydroxyprogesterone caproate has been suggested for
prophylaxis of preterm labor in women at high risk for preterm
delivery (vide infra). While prevention of preterm labor may
reduce a proportion of the patients at risk for preterm labor, it is
unlikely to eliminate preterm labor. The scoring methods for
identifying patients at high-risk for preterm labor have been found
to lack the required sensitivity. Consequently, inhibition of preterm
labor will continue to be necessary for treating those that present
with premature uterine activity.
Inhibition of Uterine Activity. While continued
intrauterine residence is in the best interest of the fetus for many
patients with preterm labor, it is necessary to decide which
pregnancies will benefit from such continuation and which ones will
fare equally well with an extrauterine existence. With the progress
and improvement made in the survival of low birth weight infants, it
may not be necessary, in patients from 35 completed weeks of
gestational age and with an estimated fetal weight of 2000 grams or
more, to have arrest of their preterm labor. Additionally, inhibition
of premature uterine activity should not be undertaken in the
presence of certain prenatal complications such as premature rupture
of the membranes, abruptio placentae, maternal genital or
intrauterine infections, severe lethal congenital malformations and
fetal death in utero.
Suppression of preterm labor may be for a short interval of up to
48 hours, to permit induction of fetal lung maturity by administering
glucocorticoids to the mother or, for longer intervals, until the
fetus is mature enough to warrant a safer extrauterine rather than
continued intrauterine existence.
The efficacy of any modality of treatment for preterm labor can
only be assessed in terms of subsequent clinical development, since
the exact mechanism triggering the onset of term and preterm labor is
incompletely understood. Therefore, there is a wide margin of error
in the accurate diagnosis of preterm labor sufficiently early enough
for treatment to be effective (see discussion on diagnosis). There is
considerable variation in the criteria adopted by individual
physicians for diagnosing preterm labor, resulting in either
overtreatment, early intervention or late intervention. Thus, success
rates are greater among those providing treatment in unindicated
cases, while those who intervene only after irreversible cervical
effacement and dilatation have occurred will obtain less impressive
results. Additionally, proper patient selection and suitable controls
will influence the efficacy rates obtained by different
investigators, while the therapeutic benefit, if any, of bed rest,
hydration and mild sedation is undefined. Because these variables can
influence the outcome of treatment, the efficacy of any modality for
inhibiting preterm labor should be evaluated with these variables in
mind. Therefore, randomized double blind controlled clinical trials,
involving a sizeable number of patients with preterm labor, are
essential in order to prove the efficacy of the various tocolytic
agents used. This is not the case with many of the agents that are
currently used. A critical analysis of 18 controlled clinical trials
on the benefit of prevention and treatment of preterm labor with
drugs, found only 13 of them methodologically
adequate.[78] In spite of the postponement of
delivery in some of these trials, only two of them favorably affected
the outcome for the infant. Thus currently used indices and end-point
for inhibition and arrest of preterm labor are not adequate enough to
provide discrimination in the ultimate outcome of the infant, which
is the final objective in the management of preterm labor.
Beta Adrenergic Receptor Stimulants (beta mimetic agents).
In 1948, Ahlquist[79] demonstrated two kinds
of adrenergic receptors, alpha and beta. Alpha receptor stimulation
usually produces vasoconstriction while beta receptor stimulation
produces vasodilatation and cardiac stimulation. Beta receptors can
be further separated into two types, beta-1 receptors, the
characteristic response of which is cardiac acceleration and
lipolysis and beta-2 receptors, stimulation of which produces
bronchial dilatation, vasodilatation and muscle glycogenolysis.
Beta-2 receptors dominate the smooth muscle of the uterus, blood
vessels, bronchioles, and diaphragm, while beta-1 are predominantly
found in the heart, small intestine and adipose tissue. Any substance
that is structurally similar to the naturally occurring
catecholamines, epinephrine and norepinephrine will stimulate beta
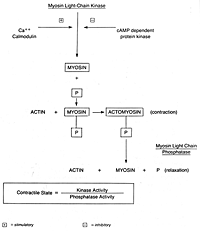
adrenergic receptors. The chemical structure of epinephrine and a
variety of beta-mimetic agents are given in Figure 3. Basically these
agents are structurally similar to epinephrine and differ only in 5.3
substitutions on the aromatic ring, the alpha and beta carbon and the
amino group. Beta receptor activity is enhanced with increase in the
size of the alkyl substitution at the amino group, while selectivity
and specificity for beta-2 adrenergic receptors are improved with
large amino group substitutions. Further selectivity and specificity
for beta-2 receptors can be enhanced by hydroxyl groups at positions
three and five of the aromatic ring and the presence of large amino
substitutions. Substitution on the alpha carbon prolongs the duration
of action of these compounds by oxidizing the monoamine oxidase
inhibitor. The beta-mimetic agents such as isoxsuprine,
orciprenaline, and isoprenaline are being overtaken by newer agents
such as fenoterol, ritodrine, salbutamol and terbutaline, all of
which are longer lasting, more specific for beta-2 receptors in the
uterine muscle and carry less serious side effects.
At the cellular level, beta-2 receptor stimulant or agonist is
coupled to adrenergic beta-2 receptor on the surface of the
myometrial cell after which adenyl cyclase is activated. Adenyl
cyclase catalyzes the conversion of adenosine triphosphate (ATP) to
cyclic adenosine monophosphate (cAMP). Cyclic AMP then increases the
activity of the enzyme protein kinase which is active in
phosphorylation of proteins in the cell membrane. This increases the
uptake and sequestration of intracellular calcium (Figure 2). The
reduction of intracellular calcium is the final mediator in
preventing activation of the contractile proteins of the muscle cell,
thus resulting in muscle relaxation.
The ideal beta adrenergic agonist would be one that mainly
influences the beta-2 receptors of the uterus with little or no
influence on the beta receptors of the heart and blood vessels, so
that minimum cardiovascular side effects will occur. However, all the
currently available beta-mimetic agents do influence beta receptors
of the heart and blood vessels to varying degrees but with clinically
observable cardiovascular side effects of variable intensity. Even
with agents that have predominantly beta-2 effects, hypotension
secondary to relaxation of the vascular smooth muscle, which is
predominantly served with the beta-2 adrenergic receptors, induces
reflex cardioacceleration with a rise in cardiac output and blood
pressure.
Ritodrine hydrochloride is a beta adrenergic stimulant with
predominant effects upon beta-2 receptors and is a potent uterine
relaxant with only minor cardiovascular and bronchial effects. The
pharmacokinetics of ritodrine are summarized in Table 2. It was
approved by the F.D.A. for use in treatment of preterm labor in the
fall of 1979 and is currently the only agent approved by the F. D.
A., among the variety of tocolytic agents that are being used for
therapy of preterm labor.
In the U.S.A., a multicenter phase III clinical evaluation of
ritodrine against ethanol and placebo for treatment of preterm labor
was carried out on 366 patients.[80] Of 313
singleton pregnancies with intact amniotic membranes, neonatal deaths
and respiratory distress syndrome were significantly reduced with
ritodrine treatment compared with control patients and pregnancy was
significantly prolonged with ritodrine (41 days) compared with
controls (24 days). [80] More pregnancies reached
greater than 36 weeks and more babies were delivered weighing more
than 2500 grams with ritodrine treatment. Prior to the multicenter
report, several of the participating investigators had reported their
results based on small groups of patients. In a European multicenter
double-blind study, ritodrine was found to be more effective in
prolonging pregnancy in preterm labor than a
placebo.[81] In another study, ritodrine was more
effective than chlordiazepoxide.[82] Other
uncontrolled studies have also suggested that ritodrine is effective
in inhibiting preterm labor.[83-86] An advantage
of ritodrine is the reduction in respiratory distress syndrome seen
in preterm infants whose mothers were treated with ritodrine,
compared with controls.[80,87]
Table 2
Pharmacokinetics of Ritodrine in Humans
|
A. Half-Lives
|
1. Oral Dose
|
First t1/2
|
1.3 hours
|
|
|
|
Second t1/2
|
12 hours
|
|
|
2. Intravenous Dose
|
First t1/2
|
6-9 mins.
|
|
|
|
Second t1/2
|
1.7-2.6 hours
|
|
|
|
Third t1/2
|
3 hours
|
|
B. Excretion
|
Orally and intravenously -- 71-93% excreted in urine
within 1 hour
|
|
C. Transplacental Passage
|
Yes - Fetal blood levels 20% that of mother
|
Ritodrine has been given intravenously, intramuscularly and orally
for treatment of preterm labor. The dose regime for the two widely
recommended routes are summarized in Table 3. The usual intravenous
dose is 50-200 mcg per minute for 24 to 48 hours and can be titrated
until uterine contractions are suppressed, after which the dose is
maintained. Oral therapy can subsequently be commenced at 10-20 mg,
4-8 times daily starting 1/2-1 hour before stopping
intravenous therapy and can be given until 36-38 weeks gestation.
The clinical side effects of ritodrine are more related to its
action as a beta-mimetic compound. Elevation of maternal heart rate,
increased pulse pressure, moderate decrease of serum potassium,
transient elevation of blood glucose and plasma insulin have been
documented. Thus the use of ritodrine in pregnant diabetics demands
careful monitoring of their blood glucose levels to avoid
ketoacidosis. Minor but frequent side effects include tremor
(10-15%), palpitations (33%), nervousness (5-10%) and restlessness
(5-10%). Side effects are generally rarely encountered during oral
therapy. Only two maternal deaths, neither of which was related to
the drug, have so far been reported in more than 480,000 patients
treated.[80] Four patients have so far developed
idiopathic pulmonary hypertension during treatment with ritodrine and
corticosteroids. Contraindications to the use of ritodrine include
overt cardiovascular disease and hyperthyroidism.
Table 3
Dose Regimen of Ritodine Hydrochloride
for Treatment of Preterm Labor
|
A. Intravenous Infusion
|
1) Initial Dose: 50-100 mcg/min.
|
|
|
2) Increase by 50 mcg/min. every 10 min. until
contractions stop or side effects occur.
|
|
|
3) Continue for 12 hours after contractions stop. 4)
Maximum dose 350 mcg/min.
|
|
B. Oral Therapy:
|
1) Initial dose-10 mg, 1/2-1 hour before
stopping IN. treatment.
|
|
|
2) 20 mg every 4 hours for 24 hours.
|
|
|
3) 10-20 mg every 4-6 hours thereafter if uterus
quiescent.
|
|
|
4) Maximum dose-120 mg/day.
|
Isoxsuprine is probably among the earliest beta-receptor agonists
which have been used for arresting preterm labor. Isoxsuprine
inhibits spontaneous and oxytocin induced uterine activity both in
vitro and in vivo during term labor.[89,90]
An efficacy rate of 72% of preterm labor being postponed by
more than seven days was reported in two uncontrolled
studies.[75,91] Uncontrolled studies have also
demonstrated the efficacy of isoxsuprine in arresting preterm
labor[92,93] but if the agent is infused only for
one hour as opposed to 4 hours, then its efficacy is
lost.[94] The usual dose of isoxsuprine for the
treatment of preterm labor is 0.25 to 0.5 mg per minute intravenously
and the rate and duration of infusion are adjusted according to
suppression of uterine activity or development of maternal
cardiovascular side effects. The patient should be placed in the
lateral supine position to minimize the hypotensive side effects.
After cessation of intravenous infusion the drug can be given
intramuscularly, 5 to 20 mg every 3 to 6 hours and then orally after
24 hours. Side effects of isoxsuprine include maternal tachycardia,
hypotension, nausea, sweating, drowsiness and headaches. An increase
of fetal heart rate by 10 to 20 beats per minute has been observed.
Terbutaline has been shown to be effective in inhibiting
myometrial contraction in vitro and in vivo during
spontaneous and oxytocin augmented labor at
term.[95,96] In two uncontrolled
studies[97.98] and one small double-blind
study,[76] terbutaline was found to be effective
in treating preterm labor in women with intact membranes. In the
double-blind study, terbutaline successfully maintained the
pregnancies to the 37th week in 80% of patients while in the placebo
group it was only 20%.[76] The dose of terbutaline
used is 10 mcg per minute initially and is increased by 5 mcg per
minute every 10 minutes up to a maximum of 25 mcg per minute or until
uterine contractions cease -- whichever occurs first. This dose is
maintained for 60 minutes once the contractions stop, after which the
maintenance dose is decreased by 5 mcg every 30 minutes to the lowest
effective dose. The lowered maintenance dose is continued for 8 hours
if uterine contractions have completely stopped. After intravenous
therapy, 250 mcg terbutaline is given intramuscularly four times a
day for three days. The oral dose of 15 mg per day is given at the
same time and maintained until 36 weeks' gestation.
Other Beta Adrenergic Agonists.
Salbutamol[99] and
fenoterol[100] were found to be useful in preterm
labor but have not been rigorously evaluated in controlled trials. In
a comparative study between buphenin, fenoterol and ritodrine, these
three agents showed no difference in the prolongation of
pregnancy.[101] Another study comparing fenoterol,
hexoprenaline, ritodrine and salbutamol found that hexoprenaline
produced the least effect on the maternal cardiovascular system but
similar effects in prolongation of pregnancy.[102]
The long term effects of beta-mimetic agents on the infant, if
any, have not been extensively looked into. In two studies, there
appears to be no significant differences in body weights of the
infants up to 18 months of age compared with
controls.[103,104] owever, both studies found some
infants with central nervous system compromise such as cerebral
palsy, intracranial hemorrhage and hemiparesis. Although the possible
long term negative effects of the beta-mimetics should be closely
monitored, it should be emphasized that preterm infants, even when
the mothers are not treated to stop the preterm birth, have a much
higher risk and incidence of such complications.
Ethanol. The use of ethanol for inhibiting human preterm
labor stems partially from studies in pregnant rabbits at term,
demonstrating that the release of bioassayable endogenous oxytocin at
parturition could be effectively inhibited by ethanol in this
species.[58] These observations have been
confirmed both during parturition and lactation using more sensitive
and specific radioimmunoassays of oxytocin.[105]
The observation that intravenous ethanol could successfully postpone
delivery in pregnant rabbits at term[106] was then
applied to the treatment of human preterm
labor.[107,108]
While the mechanisms of action of ethanol in the rabbit could be
clearly shown to be the suppression of oxytocin, the mode of action
of ethanol inhibiting human preterm uterine contractions is less well
defined. It has been proposed that the mechanism of action of ethanol
in suppressing human preterm labor is through inhibition of oxytocin
release by the neurohypophysis. Such a proposed mode of action of
ethanol has only been indirectly supported by the reduction in the
frequency of episodic release of oxytocin, when ethanol is given to
pregnant women in spontaneous labor at term.[57]
This observation is for term labor and thus the thesis that ethanol
suppresses neurohypophysial release of oxytocin in human preterm
labor is, at best, unproven. Ethanol may have some direct action on
the myometrial activity probably via a reversible interaction with
the calcium ions in the myometrial cells.[109]
The efficacy of ethanol in treating preterm labor has been
explored by several
investigators.[94,107,108,110,113] In two
uncontrolled studies of 52 and 50 human preterm labors
with intact membranes, success rates of 67-68% respectively
have been reported[107,110] but in a similar
uncontrolled study of 15 patients,
Graff[111] obtained only 7% success in postponing
delivery by 3 or more days. Similarly, in controlled studies, it has
not been the uniform experience that ethanol was more successful than
either glucose or barbiturates in postponing delivery in preterm
labor (Table 4). Indeed, except for one study showing a significantly
higher success rate with ethanol in postponing preterm
birth,[108] three subsequent studies showed no difference
between the control group and the ethanol treated group (Table
4).[94,112,113]
Such differences in success rate stem in part from differences in
the criteria for the diagnosis of preterm labor and the ethanol
dosage regime but essentially underscores the difficulty in
diagnosing early preterm labor from false labor. Although the
published data provide conflicting conclusions on the ability of
ethanol to postpone preterm births for more than 3 days, it is
generally accepted that ethanol is effective in suppressing preterm
labor for at least 24-48 hours, when the membranes are intact
and the cervix is less than 4 centimeters dilated. With
preterm rupture of the membranes, published experiences indicate
successful postponement of delivery for 24-48
hours.[107,111]
Ethanol can be given both orally and intravenously in the
treatment of preterm birth. Generally, the intravenous route is
preferable for the treatment of preterm labor, since a more steady
and constant blood level of ethanol can be readily achieved.
Nevertheless, oral administration has been and can be used at home,
if required. The protocol for the dosage of ethanol recommended in
preterm labor is given in Table 5. It is noteworthy that the maximum
length of time for administration of intravenous ethanol for preterm
labor is 12 hours and can be repeated again. Generally, it is
best to continue the ethanol infusion for several hours after
cessation of uterine contractions. It is important to emphasize that
if ethanol is administered again within 10 hours of the last ethanol
treatment, the loading or initial dose of ethanol should be adjusted
approximately as shown in Table 5. The dose of ethanol recommended
has been calculated to produce a blood level of 120-180 mg/dl.
Table 4
Controlled Studies of Ethanol in Treatment of
Preterm Labor with Intact Membranes
|
|
Control
|
Ethanol
|
Index of Success (days of
prolongation)
|
|
Authors
|
No. of Patients
|
Agent Used
|
Success (%)
|
No. of Patients
|
Success (%)
|
|
Zlatnick and Fuchs [108]
|
21
|
Dextrose
|
31
|
21
|
81
|
3
|
|
Castren, et al. [94]
|
41
|
Dextrose
|
73
|
50
|
56
|
7
|
|
Waltring, et al. [112]
|
18
|
Barbiturates
|
50
|
17
|
41
|
3
|
|
Steer and Petric [113]
|
9
|
Dextrose
|
44
|
31
|
45
|
1
|
|
Mean +/- S.E.
|
|
|
49.5 +/- 10.1
|
|
55.7 +/- 10.4
|
|
Table 5
Dose Regimen of Intravenous Ethanol
for Treatment of Preterm Labor
|
Initial Treatment
|
Loading Dose: 7.5 ml of 10% ethanol solution per kg body
weight x 2 hours
|
|
|
Maintenance Dose: 1.5 ml of 10% ethanol solution per kg
body weight x 10 hours
|
|
Repeat Treatment (less than 10 hours since last
treatment)
|
Loading Dose: 10% original dose x n (n = number of hours
since discontinuation of ethanol)
|
|
|
Maintenance Dose: As in initial treatment
|
Treatment with intravenous ethanol necessitates constant
supervision of these patients. Maternal side effects include
intoxication with blood concentrations of 100 to 150 mg/dl,
anesthesia with blood levels of 250-300 mg/dl, and coma with levels
of more than 350 mg/dl.[112,114,115] With the dose
regime of ethanol for treatment of premature labor, most women are
overtly intoxicated and usually experience nausea, vomiting, crying
and restlessness and some degree of respiratory depression. Such side
effects carry the hazards of aspiration pneumonitis. Ethanol affects
metabolism in a variety of ways. Suppression of gluconeogenesis and
significant hypoglycemia can occur, if there is depletion of hepatic
glycogen from a fasting state. Maternal lactic acidosis has been
reported"'- and dehydration with electrolyte disturbance may occur.
Hypotension and urinary incontinence, although uncommon, can occur.
Since ethanol readily crosses the placenta to reach the fetal
circulation within a minute, it is, therefore, present in the newborn
if the maternal blood ethanol levels are high. Preterm infants are at
a higher risk of developing respiratory distress
syndrome[117] and may be intoxicated at birth or
have marked suppression of the central nervous system if depressant
medications are given. Therefore, it is prudent that treatment with
ethanol be stopped if preterm labor is imminent in spite of
treatment. However, maternal treatment with ethanol appears to reduce
the risk of respiratory distress syndrome in the newborn, if the
ethanol has been eliminated by the mother and the fetus."" Two
premature infants with abnormal bone marrow morphology associated
with maternal alcohol infusion have been
reported.[119] Metabolic acidosis, muscular
hypotonia, lethargy and apnea have also been
noted.[120,121]
Contraindications to the use of ethanol for preterm labor include
patients with decompensated liver disease, maternal or fetal acidosis
and reformed alcoholics. General anesthesia and narcotic medications
should preferably be avoided when ethanol is given to stop labor.
Ethanol will shorten the half-lives of diphenylhydantoin and warfarin
and should be used cautiously in diabetic patients because of ethanol
induced hypoglycemia due to suppression of gluconeogenesis.
Prostaglandin Synthetase Inhibitor. Prostaglandins have
been shown to be synthesized and released from the decidua and fetal
membranes in spontaneous human term labor[122] and
are able to induce uterine contractions throughout pregnancy. Thus
attention has been focused on inhibiting the synthesis of
prostaglandins by the use of prostaglandin synthetase inhibitors
(PGSI) to arrest preterm labor. The mode of action of many of the
PGSI is to suppress the activity of the prostaglandin synthetase
enzymes which are necessary for the conversion of the essential
precursor fatty acid, arachidonic acid, to cyclic endoperoxides and
then to prostaglandins F and E (Figure 4). Specifically most of the
PGSI inhibit the enzyme cyclo-oxygenase which is required for the
bioconversion of arachidonic acid to cyclic endoperoxides.
Initial observations in rats showed that PGSI inhibit the
synthesis and release of prostaglandins from uterine muscle in vitro
and spontaneous uterine contractions in the rat. PGSI delayed the
onset of labor in rats, subhuman primates,[123]
and in humans.[124]
Several studies, most of which were uncontrolled, have used
indomethacin (daily oral doses of 100 mg or daily rectal doses of 200
mg), aspirin, flufenamic acid and naproxen and found that they
prolonged pregnancy in human preterm labor (Table
6).[125-133] In a small controlled study,
indomethacin given for 24 hours was shown to be significantly better
than a placebo for arresting preterm labor.[130]
In another controlled study, oral or rectal indomethacin, given over
a prolonged period of time, successfully postponed delivery by a mean
interval of 43 days in 51 patients with preterm labor, including
premature rupture of the membranes, twin gestation and quadruplet
pregnancy. In spite of the promising results in preterm labor, there
is considerable reservation about the effect of the PGSI on the
fetus. Van Kets, et a1.[132] had 3 out of 8
perinatal deaths which were possibly related to the drug among 51
preterm labor patients treated with indomethacin. One fetus was
macerated, one had Escherichia coli sepsis and the third had
an intraventricular hemorrhage with an atrial septal defect.
Table 6
Clinical Trials of Prostaglandin Synthetase
Inhibitors for Treatment of Preterm Labor
|
|
Control
|
|
|
Prostaglandin Inhibitor
|
Patient (No.)
|
Success (%)
|
Patient (No.)
|
Success (%)
|
Author
|
|
Indomethacin
|
50
|
50
|
|
|
Zuckerman et al. (1974)
|
|
|
15
|
93
|
15
|
40
|
Niebyl et al. (1980)
|
|
|
51
|
98
|
|
|
Van Kets et al. (1979)
|
|
Sodium Salicylate
|
135
|
82
|
|
|
Gyory et al. (1976)
|
|
Flufenamic Acid
|
18
|
83
|
|
|
Schwartz et al. (1978)
|
|
Naproxen
|
9
|
67
|
|
|
Wiquist et al (1977)
|
|
* Beta-mimetic agent was used before starting
prostaglandin inhibitor.
|
Indomethacin crosses the placenta freely and fetal circulatory
levels equilibrate with those of the maternal blood levels by 5 hours
after oral administration. The half-life of indomethacin in the
newborn (14.7 hours) is 7 times longer than in the nonpregnant adult
(2.2 hours).[134] Thus indomethacin may inhibit
biosynthesis of prostaglandins in fetal tissues with serious
developmental and functional sequelae. Perinatal hemorrhage due to
impaired platelet aggregation [135] and
constriction of feto-maternal vessels via contraction of the smooth
muscle are potential risks when a PGSI is used for postponing preterm
births. Other potential risks include premature closure of the ductus
arteriosus with resulting pulmonary hypertension and cardiac
failure.[136] Nevertheless, only 3 cases of
drug-related premature closure of the ductus have been
reported.[126] An infant with tricuspid
insufficiency and severe heart failure was reported after 10 days of
maternal treatment with salicylates prior to
delivery.[137] Administration of indomethacin to
the mother, up to 15 times the dose used in humans, produced
intrauterine narrowing of the fetal ductus arteriosus in
rats.[138] It appears that the gestational age
when PGSI is given to the mother is an important variable influencing
its effect on closure of the ductus arteriosus. Therefore, at
present, routine clinical use of PGSI for arresting preterm labor and
prolonging pregnancy cannot be advocated until carefully designed
double blind clinical studies, involving a substantial number of
patients, are undertaken to demonstrate both the efficacy and safety
of these agents in the treatment of labor.
Magnesium Sulfate. Magnesium sulfate decreases uterine
activity and in one study this compound was successful in arresting
preterm labor in 77% of patients compared with 45% with alcohol
treatment and 44% with dextrose solution.[113] A
potential but real side effect of magnesium sulfate is central
nervous system depression. Thus, the patients should be monitored for
loss of deep tendon reflexes and respiratory depression. It is
preferable that the magnesium sulfate is not infused for more than 24
hours, because the newborn infant may exhibit signs of
hypermagnesemia which includes loss of muscle tone and drowiness when
such prolonged administration is given.[139] The
excretion of magnesium by the newborn infant is considerably slower
than that of adults. The dose of magnesium sulfate as used by Steer
and Petrie[113] is 4 grams of 10% solution of
magnesium sulfate given intravenously as an initial loading dose. The
injection is given slowly to avoid flushing and vomiting. A
maintenance intravenous infusion dose of 2 grams per hour is then
given until uterine contractions stop or until labor becomes
irreversible. The maintenance infusion can be restarted if
contractions reappear after stopping. The ability of magnesium
sulfate to inhibit pregnant human myometrium is related to the dose
given. Magnesium sulfate concentration of 9.6 to 12.0 mg/dl will
effectively arrest uterine contractions.[140-142]
It should be emphasized that magnesium sulfate is a relatively safe
agent and its efficacy in inhibiting uterine contractions during
pregnancy makes it a suitable agent for treatment of preterm labor.
However, more adequate controlled studies in patients with preterm
labor are necessary to determine the usefulness of magnesium sulfate
in the treatment of preterm labor.
Diazoxide. Diazoxide, a powerful antihypertensive agent was
found to inhibit uterine contractions during
pregnancy.[143] Diazoxide is a benzothiadiazine
which is structurally similar to the thiazide diuretic but does not
possess its diuretic properties. It inhibits spontaneous uterine
muscle contraction in pregnant women both in vitro and in
vivo. Although diazoxide is similar to the beta-mimetic agent in
its cardiovascular effects and metabolic effects, it is not
considered a beta-adrenergic agonist because its betamimetic effects
are not blocked by beta-adrenergic blockade. Preliminary data suggest
that diazoxide can inhibit preterm labor
effectively.[144,145] However, extensive
evaluation in controlled studies has not been reported on the use of
diazoxide in preterm labor. In rats and baboons, diazoxide is able to
prolong their pregnancies.
In general, it is recommended that diazoxide be infused slowly in
low doses so as to reduce the cardiovascular effects. A dose of 300
mg intravenously over 15 minutes appears to produce only mild
cardiovascular effects. Side effects of diazoxide include lowering of
blood pressure and an increase of heart rate, cardiac output, stroke
volume, and blood flow through the coronary, femoral and renal
vessels. Urinary excretion of sodium, water, potassium chloride,
bicarbonate and uric acid are reduced but secretion of renin is
increased. Diazoxide probably inhibits insulin release and,
therefore, blood sugar is increased. Hypoglycemia may occur in the
neonate. Alopecia and hypertrichosis lanuginosa have been
noted in some infants whose mothers were given diazoxide. Diazoxide
is contraindicated in patients with compensated hypertension
(coarctation of aorta), women with coronary insufficiency, myocardial
infarction, congestive heart failure or cardiac arrhythmias.
Hormone Therapy. Progesterone and progestins suppress
myometrial contractions by elevating the excitation threshold and,
therefore, could prolong pregnancy.[146,147] On
the other hand, estrogens decrease the excitation threshold of
contraction in the myometrium. In women, there have been suggestions
that there is a fall in circulating progesterone and a rise in
estrogen at the approach or onset of labor. However, a majority of
publications in this area indicate that there is no convincing fall
in circulating progesterone levels at the approach of labor but a
rise in estrogen level is much more readily agreed upon. This
hypothesis has been extended to
premature labor and several investigators have attempted to show
either elevated circulating estrogen levels or a fall in progesterone
levels in patients with preterm
labor.[146,148,149] None of these studies have
convincingly and conclusively demonstrated that either is the case
with preterm labor in the human. Neither has the arrest of preterm
labor with any of the modalities of treatment used so far
demonstrated a reversal of the alteration in either circulating
estrogen or progesterone occurring pari passu with the
inhibition of the uterine contractions. It is thought that
restoration of progesterone dominance will result in uterine
quiescence,[146] but several double-blind control
studies using progesterone or medroxyprogesterone acetate have
uniformly failed to demonstrate any labor-suppressing effect of
progesterone in human preterm labor.[148-149] More
recently, based on a very small number of patients who were at risk
for preterm labor, it was found that 17a-hydroxyprogesterone caproate
(Delalutin) was significantly more effective than a placebo in
preventing preterm delivery.[150] If the true
incidence of preterm labor is taken into consideration, as well as
the accuracy of several risk scoring systems that have been applied
for predicting preterm labor, then it is quite obvious that any
efficacy of prophylactic treatment in preventing preterm birth in
this group of patients has to be demonstrated in a large number of
patients, so as to obviate inappropriate results from inadequate
sample size. No adverse neonatal effects were seen in the offspring
of 178-hydroxyprogesterone caproate treated patients and the drug was
given from the 16th week of pregnancy in doses of 250 mg once a week,
until the 36th week of pregnancy or until delivery. The long term
effects of such treatment on the genital tract and pituitary gonadal
axis of the offspring remain to be determined. The experience of
diethylstilbestrol on the reproductive tract of the offspring of
mothers treated with this compound should caution us against use of
hormonal agents before their efficacy and safety for a particular
purpose are well established. Finally, the dose of progesterone or
178-hydroxyprogesterone caproate is small compared to the daily
production rate of progesterone during either early or late
pregnancy. Therefore, at present, hormonal therapy to arrest preterm
labor cannot be advocated as the first line of treatment, until
incontrovertible data on its efficacy and safety are produced.
Calcium Antagonists. In preliminary studies from Europe,
calcium antagonists have been found to be effective in postponing
preterm births. In one study, ten patients with preterm labor treated
with the calcium antagonist, nifedipine, had their preterm labor
arrested and delivery was postponed by a mean interval of 14 days,
after 3 days of treatment with nifedipine.[151] In
another study the calcium antagonist was used together with a
betaadrenergic agonist. The calcium antagonist was claimed to have
reduced cardiovascular effects and may have potentiated the uterine
tocolytic effect of the beta-agonist.[152] Thus
far, there are no control studies of calcium antagonists in the
treatment of human preterm labor. No serious side effects, either on
the mother or fetus, were observed with the calcium antagonists.
Maternal heart rate may increase transiently by 10 to 25 beats per
minute.
IMMINENT PRETERM BIRTH
The mode of delivery for the preterm fetus remains controversial,
since incontrovertible data supporting either vaginal or abdominal
delivery are not readily available. The mode of delivery for the
preterm fetus is determined to some extent by the presentation
(breech versus cephalic), the fetal weight and gestational age.
Preterm Cephalic Presentation. There is even less consensus
for the optimal route of delivery in the preterm vertex presentation
compared with the preterm breech. Cesarean section significantly
improved the neonatal survival of the very low birth weight
infants,[153] but vaginal delivery also has
advantages, such as the fetal chest compression facilitating lung
expansion and the more complete placental transfusion to the fetus.
Thus it is evident that cesarean section should be more liberally
used in the delivery of the preterm vertex. Nevertheless, cesarean
section is probably more indicated in those below 30 weeks gestation,
while those infants greater than 32 weeks' gestation who carry a
lower risk of immaturity may be delivered vaginally. If vaginal
delivery is permitted, a generous episiotomy should be done and
outlet forceps liberally used to protect the less rigid fetal skull.
During labor, the preterm fetus should be monitored with
continuous fetal heart recording. It is noteworthy that the heart
rate is slightly higher with lower gestational age. Cesarean section
should be performed if there is any evidence of impending fetal
asphyxia which contributes to the development of respiratory distress
syndrome. Prophylactic use of barbiturates, analgesics and sedatives
which have been shown to have brain-protective effects against
hypoxia, by lowering cerebral metabolism, have been
recommended,[154] but others have pointed to the
adverse effect of these agents on infant
behavior.[155] At present, routine use of these
agents in preterm births cannot be recommended until more conclusive
data are available.
Preterm Breech. Breech presentation is more frequent in
preterm labor with an incidence of 25% at 28 weeks' gestation
compared with 2-3% at term. The preterm breech has a poorer perinatal
outcome, which is related to the route of delivery resulting in birth
trauma and asphyxia and also to the higher incidence of congenital
malformations. The corrected perinatal mortality of preterm breeches
is three times higher and the Apgar scores significantly lower with
vaginal delivery than with cesarean
section.[156,157] Because of this increased
neonatal morbidity and mortality, cesarean section should be
recommended for preterm births, except when major congenital
malformations that are incompatible with survival or excessively
small size infants (below 750 grams) are
found.[158] The lower limit of fetal weight to be
selected for cesarean will have to be determined according to the
neonatal mortality and survival statistics of each intensive care
nursery. Nevertheless, some workers have pointed out that cesarean
section is not invariably required for the preterm frank or complete
breech fetus.[150]
INDUCTION OF FETAL LUNG MATURATION
Many studies on human pregnancy have indicated that the
administration of glucocorticoid to the mother resulted in a
reduction of the incidence of respiratory distress
syndrome.[159-172] These studies provide
compelling evidence for the efficacy of glucocorticoid in inducing
fetal lung maturation and, therefore, reducing the risks of
respiratory distress syndrome. The effectiveness of glucocorticoid in
inducing fetal lung maturation is more obvious for pregnancies of
less than 32 weeks' gestational age, but less evident when the
pregnancy is more than 32 weeks. Besides gestational age, Apgar score
at birth and a traumatic delivery of the small preterm fetus are
important determinants in the risk of developing respiratory distress
syndrome. Glucocorticoid receptors are found in most fetal tissues
but are in greater concentration in the fetal lung. Some
glucocorticoids cross the placenta much more readily than others and
some of them have a greater affinity for binding to fetal lung
glucocorticoid receptors. In this respect, dexamethasone and
betamethasone cross the placenta readily to produce adequate
circulating levels in the fetus. Both dexamethasone and betamethasone
have a higher affinity for binding to glucocorticoid receptors in the
fetal lung and have longer plasma and biological half-lives compared
with hydrocortisone, triamcinolone and methyl prednisolone. Based on
currently available data, the administration of glucocorticoid to
induced fetal maturation is probably indicated in preterm labors
occurring before the 32nd week of gestation, if preterm birth is
imminent. Because the risk of such glucocorticoid therapy, if any,
has not been clearly defined, the use of such therapy to accelerate
fetal lung maturation in preterm labor after 32 weeks' gestation
remains debatable. The transplacental passage of betamethasone and
dexamethasone and the presence of glucocorticoid receptors in many
fetal tissues are factors that would dissuade this author from the
routine use of glucocorticoid after the 32nd week of gestation in
preterm labor, until conclusive data on its efficacy and safety are
established. If betamethasone is given, the usual dose used in most
studies has been 12 mg twice a day for 48 hours, while the dose for
dexamethasone is between 8 and 12 mg daily for up to three days.
|
|
Figure 1. High risk factors associated with preterm
delivery (Drawn from data of obstetrical statistical
cooperative study.)
|
|
|
Figure 2. Interaction of calcium with actin and myosin in
the myometrial cell to cause myometrial contraction.
|
|
|
Figure 3. Chemical structure of epinephrine and
beta-mimetic agents that can inhibit uterine contractions.
|
|
|
Figure 4. Scheme for the biosynthesis of prostaglandins
(E and F) and the site of action of prostaglandin synthetase
inhibitors.
(1) - Site of action of Type I prostaglandin synthetase
inhibitors such as indomethacin, fenamates, and ibuprofen.
(2) - Site of action of Type II prostaglandin synthetase
inhibitors such as phenylbutazone.
|
REFERENCES
1. World Health Organization: Recommendations of the WHO
Consultation of Methodology of Reporting and Analysis of Perinatal
and Maternal Morbidity and Mortality. Bristol, 1972.
2. Monthly Vital Statistics Report, United States
Department of Health, Education and Welfare, Health Resource
Administration, vol. 22, No. 7, Suppl. 7, October 2, 1973.
3. Wynn M., Wynn A.: The Prevention of Preterm Birth,
Foundation for Education and Research in Child Bearing. London,
1977.
4. Rush P. W., Davey D. A., Segall M. L.: The effect of preterm
delivery on perinatal mortality. Br. J. Obstet. Gynaecol.
85:806-811, 1978.
5. Kaltreider D. F., Kohl S.: Epidemiology of Preterm Labor
Delivery. Clin. Obstet. Gynecol. 23:17-30, 1980.
6. Boldman R., Reed D. M.: Worldwide variations in low birth
weight. In Reed D. M. and Stanley F. J. (eds.): The Epidemiology
of Prematurity. Baltimore: Urban & Schwarzenberg, 1977, p.
39.
7. Baird D.: Epidemiologic patterns over time. In Reed D. M.,
Stanley F. J. (eds.): The Epidemiology of Prematurity.
Baltimore: Urban & Schwarzenberg, 1977, p. 3.
8. Chase H. C.: Time Trends in Low Birth Weight in the United
States. In Reed D. M., Stanley F. J. (eds.): The Epidemiology of
Prematurity. Baltimore: Urban & Schwarzenberg, 1977, p. 17.
9. Pratt M. W., Janus Z. L., Sayal N. C.: Maternal Variations in
Prematurity (1973-1974). In Reed D. M., Stanley F. J., (eds.): The
Epidemiology of Prematurity. Baltimore: Urban &
Schwarzenberg, 1977, p. 53.
10. D'Angelo L., Sokol R. J.: Prematurity: Recognizing patients at
risk. Perinatal Care 2:16, 1978.
11. Van Den Berg B. J.: Epidemiologic Observations of prematurity.
Effects of tobacco, coffee and alcohol. In Reed D. M., Stanley F. J.
(eds.): The Epidemiology of Prematurity. Baltimore: Urban
& Schwarzenberg, 1977, p. 157.
12. Meyer M. B.: Effects of Maternal Smoking and Altitude on Birth
Weight and Gestation. In Reed D. M., Stanley F. J. (eds.): The
Epidemiology of Prematurity. Baltimore: Urban &
Schwarzenberg, 1977, p. 81.
13. Hardy J. B., Mellits, E. D.: Relationship of Low Birth Weight
to Maternal Characteristics of Age, Parity, Education and Body Size.
In Reed D. M., Stanley F. J. (eds.): The Epidemiology of
Prematurity. Baltimore: Urban & Schwarzenberg, 1977, p. 105.
14. Kaltreider D. F., Johnson, J. W. C. Jr.: Patients at high risk
for low birth weight delivery. Am. J. Obstet. Gynecol. 124:25,
1976.
15. Mestman J. H., Manning P. R., Hodgman J.: Hyperthyroidism and
Pregnancy. Arch. Intern. Med. 134:434, 1974.
16. Johnstone R. E., Kreindler T., Johnstone R. E.:
Hyperparathyroidism during pregnancy. Obstet. Gynecol. 40:580,
1972.
17. Grimes E. M., Fayez J. A., Miller G. L.: Cushing's Syndrome
and Pregnancy. Obstet. Gynecol. 42:550, 1973.
18. Goodlin R. C.: Orgasm and Premature Labor. Lancet
2:646, 1969.
19. Goodlin R. C., Keller D. W., Raffin M.: Orgasm during
pregnancy. Possible deleterious effects. Obstet. Gynecol.
38:916, 1971.
20. Wagner N. N., Butler J. C., Sanders J. P.: Prematurity and
orgasmic coitus during pregnancy: Data on a small sample. Fertil.
Steril. 27:911, 1976.
21. Perkins R. P.: Sexual behavior and response in relation to
complications of pregnancy. Am. J. Obstet. Gynecol. 134:498,
1979.
22. Rayburn W. F., Wilson E. A.: Coital activity and premature
delivery. Am. J. Obstet. Gynecol. 137:972-74, 1980.
23. Federick J., Anderson A. B. M.: Factors associated with
spontaneous pre-term birth. Br. J. Obstet. Gynecol. 83:342,
1976.
24. Kundsin R. B., Driscoll S. G.: Mycoplasmas and human
reproductive failure. Surg. Gynecol. Obstet. 131:89, 1970.
25. Rush R. W., Kierse M. J. N. C., Howat P., et al.: Contribution
of preterm delivery to perinatal mortality. Br. Med. J. 2:965,
1976.
26. Powers W. F.: Twin Pregnancy. Obstet. Gynecol. 42:795,
1973.
27. Tatum H. J., Schmidt F. H., Jain A. K.: Management and outcome
of pregnancies associated with the copper T intrauterine
contraceptive device. Am. J. Obstet. Gynecol. 126:869, 1976.
28. Bennett M. J., Berry J. V. J.: Preterm labour and congenital
malformations of the uterus. Ultrasound in Med. Biol. 5:83-85,
1979.
29. Benson R. C.: Premature labor and the small-for-dates infant.
In Danforth D. N. (ed.): Obstet. Gynecol. 3rd ed., Hagerstown:
Harper and Row Publishers, Inc., 1977, pp. 618-627.
30. Koh K. S.: Premature labor. Can. Med. Assoc. J.
114:700, 1976.
31. Bobitt M. R., Ledger W. J.: Unrecognized amnionitis and
prematurity. J. Reprod. Med. 19:8, 1977.
32. Liu D. T. Y., Martin T., Hudson I.: Comparative morbidity
after vaginal termination with regard to parity and gestational age.
Br. J. Clin. Pract. 28:170, 1974.
33. Wright C. S. W., Campbell S., Beazley J.: Second-trimester
abortion after vaginal termination of pregnancy. Lancet
1:1278, 1972.
34. Richardson J. A., Dixon G.: Effect of legal termination on
subsequent pregnancy. Br. Med. J. 1:1303, 1976.
35. Castracane V. D., Jordon V. C.: The effect of estrogen and
progesterone on uterine prostaglandin biosynthesis in the
ovariectomized rat. Biol. Reprod. 13:587, 1975.
36. Nissensohn R., Flouret G., Hechter O.: Opposing effects of
estradiol and progesterone on oxytocin receptors in the rabbit
uterus. Proc. Natl. Acad. Sci. (USA) 75:2044, 1978.
37. Soloff M. S.: Uterine receptor for oxytocin: effect of
estrogen. Biochem. Biophys. Res. Commun. 65:205-212, 1975.
38. Huszar G.: Current concepts of myometrial control: In
Tocolytic Agents -- Proceedings of a Symposium on Tocolytic
Therapy. New York: Biomedical Information Corporation, 1980, pp.
1-14.
39. Csapo A., Pulkkinen M.: Regnlatory and functional asymmetry of
the pregnant human uterus. Perinatol. Neonatol. 1:12, 1977.
40. Currie W. B.: Uterine excitability and distensibility
influenced by treatment in vitro with progesterone. In Ellendorf F.,
Taverne M., Smidt D. (eds.): Physiology and Control of Parturition
in Domestic Animals. Amsterdam: Elsevier, 1979.
41. Turnbull A. C., Flint A. P. F., Jeremy J. Y., et al.:
Significant fall in progesterone and rise in oestradiol in human
peripheral plasma before onset of labor. Lancet 1:7848, 1974.
42. Cousins L. M., Hobel C. J., Chang R. J., et al.: Serum
progesterone and estradiol-17b levels in premature and term labor.
Am. J. Obstet. Gynecol. 127:612, 1977.
43. Schwarz B. E., Milewich L., Johnston J. M., et al.: Initiation
of human parturition. V Progesterone binding substances in fetal
membranes. Obstet. Gynecol. 48:685, 1976.
44. Milewich L., Gant N. E., Schwarz B. E., et al.: Initiation of
human parturition VIII metabolism of progesterone by fetal membranes
of early and late human gestation. Obstet. Gynecol. 50:45,
1977.
45. Tambyraja R. L., Anderson A. S. M., Turnbull A. C.: Endocrine
changes in premature labor. Br. Med. J. 12:67, 1974.
46. Csapo A. I., Pohanka O., Kailhola H. L.: Progesterone
deficiency and premature labor. Br. Med. J. 1:137, 1974.
47. Karim S. M. M., Devlin J.: Prostaglandin content of amniotic
fluid during pregnancy and labor. J. Obstet. Gynaeeol. (Br.
Commonw.) 74:230, 1967.
48. Mitchell M. D., Flint A. P. F., Bibby J., et al.: Plasma
concentrations of prostaglandins during late human pregnancy:
Influence of normal and preterm labor. J. Clin. Endocrinol.
Metab. 46:947, 1978.
49. Nieder J., Augustin W.: Investigations on prostaglandin levels
in amniotic fluid and maternal blood during late pregnancy. Acta.
Biol. Med. Ger. 37:945, 1978. 50. Dary F., Frydman R.: Primary
prostaglandins in amniotic fluid in pregnancy and spontaneous labor.
Am. J Obstet. Gynecol. 126:13, 1976.
51. Johnson L. W. C., Dubin N. H., Calhoun S., et al.: Sequential
prostaglandin metabolite value in pregnant patients delivering at
term and preterm. International Conference on Prostaglandins,
Washington, D.C., May 1979.
52. Soloff M. S., Schroeder B. T., Chakroborty J.:
Characterization of oxytocin receptors in the uterus and mammary
gland. Fed. Proc. 36:1861, 1977.
53. Dawood M. Y., Raghavan K. S., Pociask C.: Radioimmunoassay of
Oxytocin. J. Endocrinol. 76:261-270, 1978.
54. Dawood M. Y., Raghavan K. S., Pociask C., et al.: Oxytocin in
human pregnancy and parturition. Obstet. Gynecol. 51:138-143,
1978.
55. Dawood M. Y., Wang C. F., Gupta R., et al.: Fetal Contribution
to oxytocin in human parturition. Obstet. Gynecol. 52:205-209,
1978.
56. Dawood M. Y.: Neurohypophyseal Hormones. In Fuchs F., Klopper
A. (eds.): Endocrinology of Pregnancy and Labor, 3rd ed.
Maryland: Harper and Row, 1983 (in press).
57. Gibbens G., Chard T. Observations on maternal oxytocin release
during human labor and the effect of intravenous alcohol
administration. Am. J. Obstet. Gynecol. 126:243, 1976.
58. Fuchs A-R., Wagner G.: Effect of alcohol on release of
oxytocin. Nature 198:92, 1963.
59. Lowbridge J., Manning M., Seto J., et al.: Synthetic
Antagonists of in vivo responses by the rat uterus to oxytocin. J.
Med. Chem. 22:565-569, 1979.
60. Huszar G., et al.: Pregnancy related differences in the
structure and enzymatic properties of uterine myosin. Proceedings
of the Annual Meeting of the American Society of Biological Chemists,
1980, Abstract No. 2960.
61. Symposium 1980. Phosphorylation of Muscle Contractile
Proteins. Fed. Proc. 39:1544, 1980.
62. Yagi K., et al.: Identification of an activator protein for
myosin light chain kinase as the Ca ++-dependent modulator protein.
J. Biol. Chem. 253:1338, 1978.
63. Adelstein R. S., et al.: Phosphorylation of smooth muscle
myosin in light chain kinase by the catalytic subunit of adenosine
3',5'-monophosphate-dependent protein kinase. J. Biol. Chem.
253:8347, 1978.
64. Papiernik-Berkhauer E.: Coefficient de risque d'accouchment
premature. Nouv. Presse Med. 77:793, 1969.
65. Rumeau-Rouquette C., Breart G., Deniel M., et al.: La notion
de resque en perinatologie. Resultats d'enquettes epidemiologiques.
Rev. Epidemiol. Saute Publique 24:253, 1976.
66. Fedrick J.: Antenatal identification of women at high risk of
spontaneous preterm birth. Br. J. Obstet. Gynaeeol.
83:351-354, 1976.
67. Creasy R. K., Gumner B. A., Liggins G. C.: System for
predicting spontaneous preterm birth. Obstet. Gynecol.
55:692-695, 1979.
68. Effer S., Bertola R., Vrettos A., et al.: Quantitative study
of the regularity of uterine contractile rhythm in labor. Am. J.
Obstet. Gynecol. 105:909, 1969.
69. Schulman H., Romney S.: Variability of uterine contractions in
normal human parturition. Obstet. Gynecol. 36:215, 1970.
70. Parikh M., Mehta A.: Internal cervical os during the second
half of pregnancy. J. Obstet. Gynaeeol. Br. Commonw. 68:818,
1961.
71. Anderson A., Turnbull A.: Relationship between length of
gestation and cervical dilatation, uterine contractility and other
factors during pregnancy. Am. J. Obstet. Gynecol. 105:1207,
1969.
72. Floyd W.: Cervical dilatation in midtrimester of pregnancy.
Obstet. Gynecol. 18:380, 1961.
73. Shaffner F., Schanzer S.: Cervical dilatation in the early
third trimester. Obstet. Gynecol. 27:130, 1966.
74. Hendricks C., Brennen W., Kraus G.: Normal cervical dilatation
patterns in late pregnancy and labor. Am. J. Obstet. Gynecol.
106:1065, 1970.
75. Csapo A. I., Herczeg J.: Arrest of premature labor by
isoxsuprine. Am. J. Obstet. Gynecol. 129:182, 1977.
76. Ingemarsson I.: Effects of terbutaline on premature labor.
Am. J. Obstet. Gynecol. 125:520, 1976.
77. Whitfield C., Sproule W.: Fetal lung maturation. Br. J.
Hosp. Med. 12:678, 1974.
78. Hemminki E., Starfield B.: Prevention and Treatment of
Premature Labor by Drugs: Review of Controlled Clinical Trials.
Br. J. Obstet. Gynaecol. 85:411417, 1978.
79. Ahlquist R. P. P.: A study of the adrenotropic receptors.
Am. J. Physiol. 153:586, 1948.
80. Merkatz I., Peter J. B., Barden T. P.: Ritodrine
Hydrochloride: A Betamimetic Agent for use in Preterm Labor II.
Evidence of Efficacy. Obstet. Gynecol. 56:7-13, 1980.
81. Wesselius-de Casparis A., Thiery M., Yo Le Sian A., et al.:
Results of doubleblind, multicentre study with ritodrine in premature
labour. Br. Med. J. 3:144, 1971.
82. Sivasamboo R.: Premature labour. In Baumgarten K. and
Wesselius-de Casparis A. (eds.): Proceedings of the International
Symposium in the Treatment of Fetal Risk. Baden, Austria:
University of Vienna, 1972, p. 16.
83. Decelle J., Vokaer R.: A clinical trial with ritodrine in
threatened premature labour. In Baumgarten K. and Wesselius-de
Casparis A. (eds.): Proceedings of the International Symposium on
the Treatment of Fetal Risk. Baden, Austria: University of
Vienna, 1972, p. 36.
84. Renaud R., Irrmann M., Flynn M., et al.: The treatment of
premature labour with ritodrine hydrochloride. In Baumgarten K. and
Wesselius-de Casparis A. (eds.): Proceedings of the International
Symposium in the Treatment of Fetal Risk. Baden, Austria:
University of Vienna, 1972, p. 42.
85. Thiery M., Baumgarten K., Brosens I., et al.: A multicentre
trial with ritodrine in the treatment of premature labour in patients
with intact membranes. In Baumgarten K. and Wesselius-de Casparis A.
(eds.): Proceedings of the International Symposium in the
Treatment of Fetal Risk. Baden, Austria: University of Vienna,
1972, p. 21.
86. Bieniarz J., Motew M., Scommegna A.: Uterine and
cardiovascular effects of ritodrine in premature labor. Obstet.
Gynecol. 40:65, 1972.
87. Esteban-Altirriba J.: Fetal Lung Maturation: In tocolytic
Agents. Proceedings of a Symposium on tocolytic therapy. New
York: Biomedical Information Corporation, 1980, p. 41-48.
88. Barden T. P., Peter J. B., Merkatz L: Ritodrine hydrochloride:
A Beta-mimetic Agent for use in Preterm Labor. Obstet. Gynecol.
56:1-5, 1980.
89. Lish P. M., Hillyard I. W., Dungan K. W.: The uterine relaxant
properties of isoxsuprine. J. Pharmacol. Exp. Ther. 129:438,
1960.
90. Eriksson G., Wiqvist N.: Action of isoxsuprine and its (+)
isomer on the pregnant human uterus. Am. J. Obstet. Gynecol.
91:1076, 1965.
91. Das R. K.: Isoxsuprine in premature labor. J Obstet Gynaecol
India 19:566, 1969.
92. Bishop E. H., Woutersz T. B.: Arrest of premature labor. J.
Am. Med. Assoc. 178:116, 1961.
93. Hendricks C. H.: The use of isoxsuprine for the arrest of
premature labor. Clin. Obstet. Gynecol. 7:687, 1964.
94. Castren O., Gummerus M., Sasrikoski S.: Treatment of imminent
premature labour. Acta Obstet. Gynaecol. Scand. 54:95, 1975.
95. Andersson K. E., Ingemarsson I., Persson C. G. A.: Effects of
terbutaline on human uterine motility at term. Acta Obstet.
Gynaecol. Scand. 54:165, 1975.
96. Andersson K. E., Bengtsson L., Gustafson L, et al.: The
relaxing effect of terbutaline on the human uterus during term labor.
Am. J. Obstet. Gynecol. 121:602, 1975.
97. Garcia C. V., Garcia M. J., Ahued R., et al.: Terbutaline, un
nuevo uteroinhibidor. Gynecol. Obstet. Mex. 36:75, 1974.
98. Wallace R. L., Caldwell D. L., Ausbacher R., et al.:
Inhibition of premature labor by terbutaline. Obstet. Gynecol.
51:387, 1978.
99. Liggins G. C., Vaughn G. S.: Intravenous infusion of
salbutamol in the management of premature labor. J. Obstet.
Gynaecol. Brit. Commonw. 80:29, 1973.
100. Lippert T. H., DeGrandi P. B., Fridrich R.: Actions of the
uterine relaxant, fenoterol on utero placental hemodynamics in human
subjects. Am. J. Obstet. Gynecol. 125:1093, 1976.
101. Richter R.: Evaluation of success in treatment of threatening
premature labor by Betamimetic drugs. Am. J. Obstet. Gynecol.
127:482, 1977.
102. Lipschitz J., Baillie P., Davey D. A.: A comparison of the
uterine beta 2-adreno receptor selectivity of fenoterol,
hexoprenaline, ritodrine and salbutamol. S. Afr. Med. J.
50:1969, 1976.
103. Freysz H., Willard D., Lehr A., et al.: A long term
evaluation of infants who received a Betamimetic drug while in utero.
J. Perinat. Med. 5:94, 1977.
104. Karlsson K., Drantz M., Hamberger L.: Comparison of various
betamimetics on preterm labor, survival and development of the child.
J. Perinat. Med. 8:19-26, 1980.
105. Fuchs A-R., Dawood M. Y.: Oxytocin release and uterine
activation during parturition in rabbits. Endocrinology
107:1117, 1980.
106. Fuchs A-R.: The inhibitory effect of ethanol on the release
of oxytocin during parturition in the rabbit. J. Endocrinol.
35:125, 1966.
107. Fuchs F., Fuchs A-R., Poblete V., Jr. et al.: Effect of
alcohol on threatened premature labor. Am. J. Obstet. Gynecol.
99:627, 1967.
108. Zlatnik F. J., Fuchs F.: A controlled study of theanol in
threatened premature labor. Am. J. Obstet. Gynecol. 112:610,
1972.
109. Fuchs A-R., Fuchs F.: Possible mechanism of the inhibition of
labor by ethanol. In Josimovich J. B. (ed.): Uterine Contraction.
New York: John Wiley and Sons, 1973.
110. Mehra P., Raghavan K. S., Devi P. K., et al.: Effect of
intravenous alcohol on premature labour. Int. J. Gynaecol. Obstet.
8:160, 1970.
111. Graff G.: Failure to prevent premature labor with ethanol.
Am. J. Obstet. Gynecol. 110:878, 1971.
112. Watring W., Bensen W., Wiebe R., et al.: Intravenous
alcohol-A single blind study in the prevention of premature delivery:
A preliminary report. J. Reprod. Med. 16:35,1976.
113. Steer C. M., Petrie R. H.: A comparison of magnesium sulfate
and alcohol for the prevention of premature labor. Am. J. Obstet.
Gynecol. 129:1, 1977.
114. Ritchie J. M.: The aliphatic alcohols. In Goodman L. S. and
Gilman A. (eds.): The Pharmacological Basis of Therapeutics,
5th ed. New York: Macmillan Publishing Company, Inc., 1975, pp.
137-151.
115. Isselbacher K. J.: Metabolic and hepatic effects of alcohol.
N. Engl. J. Med. 296:612, 1977.
116. Ott A., Hayes J., Polin J.: Severe lactic acidosis associated
with intravenous alcohol for premature labor. Obstet. Gynecol.
48:362, 1976.
117. Zervoudakis I., Krauss A., Fuchs F.: Infants of mothers
treated with ethanol for premature labor. Am. J. Obstet. Gynecol.
137:713, 1980.
118. Barrada M. L, Virnig N. L., Edwards L. E., et al.: Maternal
intravenous ethanol in the prevention of respiratory distress
syndrome. Am. J. Obstet. Gynecol. 129:25, 1977.
119. Lopez R., Montoya M.: Abnormal bone marrow morphology in the
premature infant associated with maternal alcohol infusion. J.
Pediat. 79:1008, 1971.
120. Idanpaan-Heikkila J., Jouppila P., Akerblom H. K., et al.:
Elimination and metabolic effects of ethanol in mother, fetus and
newborn infant. Am. J. Obstet. Gynecol. 112:387, 1972.
121. Mann L., Bhakthavathsalan A., Liu M., et al.: Placental
transport of alcohol and its effect on maternal and fetal acid-base
balance. Am. J. Obstet. Gynecol. 122:837, 1975.
122. MacDonald P. C., Schultz F. M., Duenhoelter J. H., et al.:
Initiation of human parturition. Obstet. Gynecol. 44:629,
1974.
123. Novy M. J., Cook M. J., Manaugh L.: Indomethacin block of
normal onset of parturition in primates. Am. J. Obstet.
Gynecol. 118:412, 1974.
124. Lewis R. B., Schulman J. D.: Influence of acetylsalicylic
acid, gestation and labour. Lancet 2:1159, 1973.
125. Zuckerman H., Reiss U., Rubinstein L: Inhibition of human
premature labor by indomethaein. Obstet. Gynecol. 44:787,
1974.
126. Wiqvist N., Lundstrom V., Green K.: Premature labor and
indomethaein. Prostaglandins 10:515, 1975.
127. Babenerd J., Kyriakidis K.: Wehemmung durch
Azetylsalizylsaure. Fortschr. Med. 97:463, 1979.
128. Gyory G., Kurez K. M., Benyo F., et al.: Klinische Anwendung
der Prostaglandin Antagonisten in der Gebrutshilfe. Zentbl.
Gynaekol. 98:1263, 1976
129. Schwartz A., Brook I., Insler V., et al.: Effect of
flufenamic acid on uterine contractions and plasma levels of
15-keto-13, 14-dihydro PGF2a in preterm labor. Gynecol. Obstet.
Invest. 9:139, 1978
130. Niebyl J. R., Blake D. A., White R. D., et al.: The
inhibition of premature labor with indomethaein. Am. J. Obstet.
Gynecol. 136:1014-9, 1980.
131. Atad J., David A., Moise J., et al.: Classification of
threatened premature labor related to treatment with a prostaglandin
inhibitor: Indomethacin. Biol. Neonat. 37:291-296, 1980.
132. van Kets H., Thiery M., Deram R., et al.: Perinatal hazards
of chronic antenatal tocolysis with indomethaein. Prostaglandins
18:893, 1979.
133. Spearing G.: Alcohol, indomethaein and salbutanol. Obstet.
Gynecol. 53:171, 1979.
134. Traeger V. A., Nosehell H., Zaumseil J.: Pharmakokinetic von
indomethaein bei Schwangeren, Kreibenden and deren Neugeborenen.
Aentralbl. Gynaekol. 95:635, 1973.
135. Haslam R. R., Ekert H., Gillam G. L.: Hemorrhage in a neonate
possibly due to maternal ingestion of salicylate. J. Pediat.
84:556, 1974.
136. Coceani F., Olley P. M., Bodach E.: Prostaglandins: a
possible regulator of muscle tone in the ductus arteriosus. In
Samuelsson B., Paoletti R. (eds.):Advances in Prostaglandin and
Thromboxane Research, vol. l. New York: Raven Press, 1976, p.
417.
137. Areilla R. A., Thilenius O. G., Ranniger K.: Congestive heart
failure from suspected ductal closure in utero. J. Pediat.
75:74, 1969.
138. Sharpe G. L., Larsson S., Thalme B.: Studies on closure of
the ductus arteriosus. XII. In utero effect of indomethaein and
sodium salicylate in rats and rabbits. Prostaglandins 9:585,
1975.
139. Lipsitz P. J.: The clinical and biochemical effects of
magnesium in the newborn. Pediatrics 47:501, 1971.
140. Kumar D., Zourtlas P. A., Barnes A. C.: In vitro and in vivo
effects of magnesium sulfate on human uterine contractility. Am.
J. Obstet. Gynecol. 86:1036, 1963.
141. Hutchinson H. T., Nichols M. M., Kuhn C. R., et al.: Effect
of magnesium sulfate on uterine contractility, intrauterine fetus,
and infant. Am. J. Obstet. Gynecol. 88:787, 1964.
142. Harbert G. M., Cornell G. W., Thornton W. N. Jr.: Effect of
toxemia therapy on uterine dynamics. Am. J. Obstet. Gynecol.
105:94, 1969.
143. Finnerty F. A., Kakaviatos N., Tuckman J., et al.: Clinical
evaluation of diazoxide. Circulation 28:203, 1963.
144. Barden T. P., Keenan W. J.: Effects of diazoxide in human
labor and the fetus-neonate. Obstet. Gynecol. 37:631, 1971.
(Abst.)
145. Bert J. J.: Tocolytic properties of diazoxide. Presented at
the Fifth International Conference on Birth Defects. Montreal,
Quebec, Canada, August, 1977. (Abst. 190)
146. Csapo A. L: The regulatory interplay of progesterone
prostaglandin F2a in the control of the pregnant uterus. In
Josimovich J. B. (ed.): Uterine Contraction. New York: John
Wiley and Son, Inc., 1973, pp. 233-255.
147. Daniel E. E., Lodge S.: Electrophysiology of myometrium. In
Josimovich J. B. (ed.): Uterine Contraction. New York: John
Wiley and Sons, Inc., 1973, pp. 19-64.
148. Fuchs F., Stakemann G.: Treatment of threatened premature
labor with large doses of progesterone. Am. J. Obstet. Gynecol.
79:172, 1960.
149. Brenner W. E., Hendricks C. H.: Effect of medroxyprogesterone
acetate upon the duration and characteristics of human gestation and
labor. Am. J. Obstet. Gynecol. 83:1094, 1962.
150. Johnson J. W. C., Austin K. L., Jones G. S., et al.: Efficacy
of 17a hydroxyprogesterone caproate in the prevention of premature
labor. N. Engl. J. Med. 293:675, 1975.
151. Ulmsten U., Andersson K-E., Wingerup L.: Treatment of
Premature labor with the calcium antagonist nifedipine. Arch.
Gynecol. 229:1-5, 1980.
152. Mosler K. H.: The treatment of threatened premature labor by
tocolytics, Ca++ antagonists and inflammatory drugs. Arzneim
Forsch. 25:263, 1975.
153. Stewart A. L., Turcan D. M., Rawlings G., et al.: Prognosis
for infants weighing 1000g or less at birth. Arch. Dis. Child.
52:97, 1977.
154. Myers R. E., Myers S. E.: Use of sedative, analgesic and
anesthetic drugs during labor and delivery: Bane or boon. Am. J.
Obstet. Gynecol. 133:83, 1979. 155. Conway E., Brackbill Y.: II.
Delivery medication and infant outcome. An empirical study. Soc.
Res. Child. Devel. 35:24, 1970.
156. Goldenberg R. J., Nelson K. G.: The premature breech. Am.
J. Obstet. Gynecol. 127:240, 1977.
157. Lyons E. R., Papsin F. R.: Cesarean section in the management
of breechpresentation. Am. J. Obstet. Gynecol. 130:588, 1978.
158. Karp L. E., Soney J. R., McCarthy T., et al.: The premature
breech: trial of labor or cesarean section? Obstet. Gynecol.
53:88, 1979.
159. Fargier P., Salle B., Baud M., et al.: Prevention du syndrome
de detresse respiratoire chez la premature. Interet du traitement
antepartum par less glucoorticoides. Nouv. Presse Med. 3:1595,
1974.
160. Kennedy J. L. Jr.: Antepartum betamethasone in the prevention
of respiratory distress syndrome. Pediatr. Res. 8:173, 1974.
161. Bureau M., Stocker J., Deleon A., et al.: Utilisation de la
betamethasone dans la prevention du syndrome de detresse respiratoire
due noveau-ne premature. Union Med. Can. 104:99, 1975.
162. Kennedy J. L. Jr.: Clinical experience with betamethasone for
the prevention of respiratory distress syndrome. In Moore T. D.
(ed.): Lung Maturation and the Prevention of Hyaline
Membrane Disease. Report of the 70th Ross Conference on Pediatric
Research. Columbus, Ohio: Ross Laboratories, 1976, p. 105.
163. Liggins G. C.: Prenatal Glucocorticoid treatment: prevention
of respiratory distress syndrome. In Moore T. D. (ed.): Lung
Maturation and the Prevention of Hyaline Membrane Disease.
Proceedings of the 70th Ross Conference on Pediatric Research.
Columbus, Ohio: Ross Laboratories, 1976, p. 97.
164. Dluholucky S., Babic J., Taufer L: Reduction of incidence and
mortality of respiratory distress syndrome by administration of
hydrocortisone to mother. Arch. Dis. Child. 51:420, 1976.
165. Caspi E., Schreyer P., Weinraub Z., et al.: Prevention of the
respiratory distress syndrome in premature infants by antepartum
glucocorticoid therapy. Br. J. Obstet. Gynecol. 83:187, 1976.
166. Block M., Kling O., Crosby W.: Antenatal glucocorticoid
therapy for the prevention of RDS in the premature infant. Obstet.
Gynecol. 50:186, 1977.
167. Morrison J. C., Whybrew W. D., Bucovac E. T., et al.:
Injection of corticosteroids into mother to prevent neonatal
respiratory distress syndrome. Am. J. Obstet. Gynecol.
131:358, 1978.
168. Thomfeldt R. E., Franklin R. W., Pickering N. A., et al.: The
effect of glucocorticoids on the maturation of premature lung
membranes. Am. J. Obstet. Gynecol. 131:143, 1978.
169. Ballard R. A., Ballard P. L., Granberg J. P., et al.:
Prenatal administration of betamethasone for prevention of
respiratory distress syndrome. J. Pediatr. 94:9, 1979.
170. Papageorgiou A. N., Desgranges M. F., Masson M., et al.: The
antenatal use of betamethasone in the prevention of respiratory
distress syndrome: a controlled double-blind study. Pediatrics
63:73, 1979.
171. Quirk J. G. Jr., Raker R. K., Petrie R. H., et al.: The role
of glucocorticoids, unstressful labor, and atraumatic delivery in the
prevention of respiratory distress syndrome. Am. J. Obstet.
Gynecol. 134:768, 1979.
172. Taeusch H. W., Frigoletto F., Kitzmiller J., et al.: Risk of
respiratory distress syndrome after prenatal dexamethasone treatment.
Pediatrics 63:64, 1979.
Return to the Table of Contents Page
Created 9/13/2002 / Last modified 9/13/2002
Neonatology on the Web / webmaster@neonatology.net



