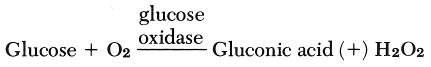
INTRODUCTION
Biochemical monitoring of patients took its roots during the early 1900's. In the beginning, the procedures were based mainly on chemical analysis. Then, the colormetric method came into use. The use of photoelectric cells was introduced in the 1930's, followed by the introduction of flame photometers and spectrophotometers. However, the problems of biochemical analysis lay in the limitations on the quantities of blood that could be used. New techniques to analyze small quantities of blood were described in the 1920's. Since then, many methods have been published describing micromethods that were simple, reliable and accurate. While these tests were developed to analyze smaller amounts of blood, the quantity required per test was not small enough for the pediatric population in general and for neonates in particular. As a result, ultra micromethods were developed in the 1960's and today the samples required for analysis of an individual test do not exceed 0.05 ml.[1,2]
In current practice, reliable laboratory support is an integral part of any Intensive Care Unit. The newborn population in general, and premature and sick babies in particular, have even more demanding laboratory requirements. The special nature of this group requires significant differences in the way laboratory tasks are performed. Sensitive and accurate micromethods that utilize minimum volumes of serum or other specimens are required for this purpose. The goal of the neonatal intensive care laboratory should be to perform the most commonly requested tests using a very small sample to avoid iatrogenic anemia. Development of such methods is a growing part of laboratory medicine.
Laboratory support is required for proper management of a sick baby. For example, a newborn on a respirator requires innumerable blood-gas determinations and other processes around the clock. For this reason, most blood-gas laboratories are located as close as possible to, and are readily accessible to, the neonatal care nursery on a 24 hour-a-day and 7 day-a-week basis.
The most common tests requested of the microchemistry laboratories by the Neonatal Intensive Care Unit (NICU) are:
Special tests less frequently requested:
In addition, although less frequently requested, such laboratories must have access to a metabolic laboratory for amino acid analysis and ammonia and lactate determination to diagnose inborn errors of metabolism[3] early in life to prevent irreversible brain damage.
Blood samples for newborn screening must also be collected on a filter paper before discharge from the nursery and sent to the appropriate laboratory, with follow-ups if necessary. Also, documentation must be in the record. The number of disorders that each state mandates be screened varies. In Illinois, all newborn infants must be screened for hypothyroidism and PKU.
The laboratory for neonatal intensive care is specialized and expensive, but the medical care and management of sick and/or premature babies, which must be based on accurate laboratory data, cannot be compromised and, with increased survival of very immature infants, the need for such a laboratory cannot be over-emphasized.
The points of concern in a neonatal intensive care laboratory, in which the patient population includes newborn and premature infants, are the subjects of this chapter. These concerns include proper collection of specimens, choice of methodology, instrumentation reference values and the urgency of reporting.
SPECIAL PROBLEMS OF OBTAINING PROPER SPECIMENS FROM NEWBORNS
The special anatomical and physiological characteristics of the infant, its blood volume, the baby's limited compensatory mechanisms, the alteration in its disease patterns and the volatility of its responses to stress must be taken into account in specimen collection. These characteristics demand strict controls on the collection and handling of the specimen.
Newborn blood specimens can be obtained by skin puncture, venipuncture, arterial puncture and through umbilical artery or venous catheters. Regardless of the collection method, the major considerations in collecting blood from infants are: (1) the volume of the specimen, (2) the potential for injury to the infant, (3) the infection potential, and (4) the various thromboembolic complications secondary to the use of catheters.
Heel puncturing is the least hazardous technique for collecting neonatal blood samples. New clinical laboratory analyzers require only microquantities of serum or plasma for multiple tests. The small amounts could easily be collected by heel puncture in heparinized capillary tubes. Heel pricks must be done cautiously and the depth must not exceed 2.4 mm to avoid injury to underlying bone. A free blood flow is necessary to avoid hemolysis and contamination with cellular fluid. When collecting blood from UA or UV catheters, avoid using the first 2 cc of blood to prevent errors due to dilution.
NORMAL REFERENCE VALUES
The reference values which are defined as the amount of a substance present in the serum, urine or other body fluid of a healthy individual, vary over a range. A number of factors, such as age, sex, diet and genetic constitution, will affect these values. Therefore, it is imperative that normal reference values be established for neonates and premature infants. The values given in Table 1 are taken from our own accumulated results and they apply to the methods of analysis used in our laboratory.
Normal reference values must be established for neonates and premature infants in each laboratory, since significant differences may exist between adults and newborns, and even from one laboratory to another.
Normal Values and Ranges
|
Test |
Normal |
|
Bilirubin (total) |
< 1.2 mg/dl |
|
Bilirubin (direct) |
< 0.2 mg/dl |
|
Calcium |
5.0 ± 0.5 meq/1 |
|
Chloride |
103. ± 5 meq/1 |
|
Glucose |
87 ± 22 mg/dl |
|
Potassium |
4.4 ± 0.9 meq/1 |
|
Sodium |
142 ± 7 meq/1 |
|
Blood Gases |
|
|
pH |
7.36-7.44 |
|
PCO2 |
34-46 mm Hg |
|
PO2 |
|
|
Venous |
32 ± 7 mm Hg |
|
Arterial |
85 ± 5 mm Hg |
|
Base Excess |
2.3 to -2.3 meq/1 bl |
|
Total CO2 |
23-27 meq/1 plasma |
|
Actual Bicarb |
22-26 meq/1 plasma |
|
Standard Bicarb |
21.3-24.8 meq/1 plasm |
|
Buffer Base |
Depends on HB level (meq/blood) |
|
Hemoglobin |
|
|
Male |
14-18 g/dl |
|
Female |
12-16 g/dl |
QUALITY CONTROL
It must be emphasized that the establishment of reference values is useful only if it is done accurately. To ensure the accuracy of values obtained, it is now a standard practice for any laboratory to monitor its own performance by subscribing to one or more of the available comprehensive proficiency testing programs. Our laboratory utilizes Dades' Labtrol (Scientific Products, McGaw Park, Illinois) Patharol and Bilirubin controls. These controls are run with each set of tests. Should the controls for any of the tests be an "outlier" the reason must be sought and corrected before tests are repeated.
STAFF
The laboratory should be supervised by a responsible clinical chemist, biochemist or a clinician well versed in laboratory medicine. Adequate staff of high caliber should be employed to ensure that the laboratory is covered 24 hours a day, seven days a week with well trained and competent technologists. Communication among the laboratory staff, the neonatologists and the house staff is the most important aspect of good service. Participation of senior laboratory chemists in the medical rounds, along with the neonatologists and house staff, is helpful in learning why tests are ordered, how results are interpreted and what additional tests are available which can be useful.
It is also important for technologists to participate in weekly conferences where case histories are discussed so that they can regard themselves as an integral part of the medical team and understand their role in the health care system. Four or five full-time equivalent persons are required in order to give 24 hour service to a microchemistry laboratory.
INSTRUMENTS
There is a variety of instruments that are used in intensive care laborato-ries The main prerequisite is the sample size. As stated earlier, the goal of a laboratory should be to perform most of the commonly requested tests with a minimum volume of serum or other specimen. The procedure should be specific for the test substance such as the glucose oxidase method for the determination of glucose levels.
The following is a description of procedures and instruments we have found suitable for our laboratory:
Glucose analysis. The estimation of serum glucose is important with respect to very low levels as well as very high levels, because hypoglycemia and hyperglycemia occur commonly in neonates, requiring repeated and rapid determination of blood glucose levels. Bedside evaluation can be done by using dextrostix. However, for quantitative determination of blood glucose levels a glucose analyzer is used.
The Glucose Analyzer (Beckman Instruments, Fullerton, California) uses an oxygen sensor electrode and an electronic system that measures the rate of change in oxygen concentration. When a sample is injected into an enzyme reagent solution, Beta-D glucose in the sample combines with dissolved oxygen from the solution according to this reaction:
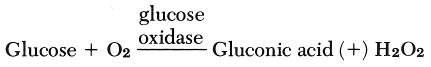
The rate of oxygen consumption in the above reaction (A) is the same rate at which glucose reacts and is directly proportional to glucose concentration.
Bilirubin analysis. Bilirubin determination constitutes the largest number of tests ordered in neonatal service. The instrument used is a bilirubinometer (Advanced Instruments, Needham, Massachusetts) which is essentially a double spectrophotometer with wave lengths fixed at 454 nm and 540 run. The instrument requires .30 ml of serum for analysis and is best suited for determination of bilirubin in newborn infants. Direct and total bilirubin is determined by this instrument.
Chloride titration. The CMT 10 chloride (Radiometer, Copenhagen, Denmark) titrator is used specifically for the determination of chloride content in the serum. During titration, silver ions are generated at the silver anode, located at the bottom of the titration vessel. The end point is detected potentiometrically by an electrode pair comprising a silver indicator electrode and mercury-mercurous sulphate reference electrode. The instrument needs 10 ml of serum.
Electrolyte determination. Fluid and electrolyte balance in sick newborns is of great concern, so innumerable electrolyte determinations are requested daily.
The flame photometer (Beckman Instruments, Fullerton, California) provides a fast and accurate means for determining sodium and potassium concentration.
A sample responds optically and electronically to the intensity of the principle emission lines that characterize sodium and potassium as each is excited in the oxygen propane flame. The flame is monitored continuously by three photomultiplier detectors. The sodium detector responds only to wavelengths in a narrow band centered at 591 millimicrons; the potassium detector responds only to wavelengths in a narrow band centered at 768 millimicrons; the lithium detector responds only to wavelengths in a narrow band centered at 671 millimicrons.
The diluted sample containing the fixed known amount of lithium salt is nebulized. The fine mist of the sample is dispersed in the spray chamber, mixed with propane and carried up to the burner where it is ignited in the presence of air generated by a compressor.
The lithium concentration in the standards and the samples is fixed. The instrument "reads" the ratio between the sodium and potassium characteristics of a sample and the characteristics of the known amount of lithium. Any changes in the emission lines that might occur because of fluctuation in air or fixed pressure or sample aspiration ratio will affect equally the sample characteristics and the lithium standard characteristic. The ratio of lithium in the standards and samples must remain constant.
Along with chloride, abnormalities of Na and K are commonly seen in newborns, especially in extremely premature babies.
Calcium determination. Monitoring calcium levels is equally important and low levels are frequent occurrences in preterm babies, asphyxiated newborns and infants of diabetic mothers as a result of hypoparathyroidism. This could be hazardous to life and could affect preterm babies, especially those in an active growth period.
Blood gas determination. Repeated blood gas determinations are necessary in critically ill neonates. Blood gas analysis requires 100 microliters of arterial blood or one capillary blood gas tube (approximately 140 microliters if drawn from the heel). Each specimen requires a hematocrit determination. Capillary pH and PCO2 correlate well with arterial levels, but it is not always so with PO2 levels.
Most often, for routine blood gas analysis, a heel puncture specimen is used for which the heel should be hyperemic. In cases of shock, acidosis, or hypothermia, capillary blood gases are not reliable. Arterial blood is necessary if the newborn patient is in respiratory distress when accurate assessment of oxygenation is necessary. Arterial blood is also necessary for diagnosing hyperoxia (i.e. PO2 > 100 mm Hg) for prevention of retrolental fibroplasia where capillary levels are unreliable.
Our laboratory employs a BMS3 Mark 2 (Radiometer, Copenhagen, Denmark) for blood gas determinations. Complete calibration of the gas and pH electrodes is performed several times a day to ensure that the zero and the span of the meter are in agreement with the zero and the sensitivity of the electrodes. The pH electrode is checked with appropriate buffers to ensure that the electrode is operative and undisturbed by excessive drift.
The Siggaard-Anderson Alignment Monogram is used for calculation of base excess, bicarbonate and total CO2 after pH, PCO2 and hemoglobin have been measured. On the other hand if pH, total CO2 and hemoglobin are measured, PCO2, base excess and bicarbonate can be read from the monogram. In cases of severe metabolic acidosis, calculation of the base deficit is helpful for infusion of sodium bicarbonate.
Our laboratory uses a Corning 940 Calcium Analyzer (Corning Scientific Instrument, Medfield, Mass.) The instrument is based on the Complexometric titration of calcium with the calcein, a fluroscein derivative. Calcein forms an intensely fluorescent, non-dissociated complex with calcium. The quenching of this fluorescence by chelating the calcium with the titrant elthylene glycol bis (b amino ethyl ether) N, N-tetra-acedic acid (EGTA) is then measured. The titration stops when all the calcium has been chelated by the EGTA and a predetermined level of fluorescence is attained. The analyzer is equipped with a fluorometer which automatically defines the endpoint. An electronic calculator then digitally displays the calcium concentration.
The analyzer's range is 3.0 mg/dl to 19.99 mg/dl for serum and plasma, and 0.00 mg/dl to 18.00 mg/dl for urine. The analyzer requires 10 microliters of serum for analysis.
SPECIAL TESTS
Although less common, a newborn with urea cycle defects or organic acidemias may require serial blood ammonia determinations. A variety of chemical methods is available for measurement of the ammonia content of plasma. We use a quantitative ultraviolet procedure in our laboratory. This method avoids the use of alkali, which may result in the liberation of amines as well as the formation of ammonia by deamination of acid amides or by deamination reaction.
The method is based on reductive amination of a-ketoglutrate using glutamate dehydrogenase and reduced nicotinamide adenine dinucleotide (NADH).
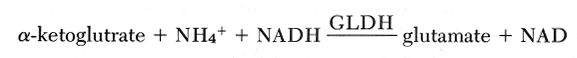
The enzyme is specific for NH4 + and will not react with methylated amines. The decrease in absorbance at 340 nm, due to oxidation of NADH, is proportional to the concentration of ammonium in the plasma. This change in absorbance is measured in a Beckman DU-spectrophotometer. Ammonia-free heparin is used as anticoagulant and hemolysis should be avoided. Upon drawing, the blood should be placed in an ice bath and plasma should be separated within 30 minutes.
The reagents for the determination of ammonia and a pamphlet describing the procedure is available in a kit form from Sigma Chemical Company, St. Louis, Missouri (Sigma Technical Bulletin No. 170-UV).
Hyperammonemia can be fulminant in the first few days of life. The attack is often precipitated by the first protein feeding and produces lethargy, poor feeding, vomiting, hypotonia, seizures and coma. Hyperammonemia is a feature of disorder of enzymes of the urea cycle and disorders of dibasic branched chain amino acid metabolism. Nongenetic causes of hyperammonemia are rare, but include any cause of severe liver failure due to hepatitis or sepsis of hypoxia and occur from hyperalimentation.
Amino acid analysis. The amino acid pattern in the urine can be analyzed by several methods. These include one- or two-dimensional chromatography on paper, thin layer plates, or electrophoretic techniques. The problem of interpretations is complicated by the fact that renal tubular reabsorption of amino acids is much less complete in newborns. Generalized aminoaciduria can occur in premature babies and can be produced by many drugs and systemic illness. In suspected cases, amino acid should be analyzed in plasma. Furthermore, urinary and plasma amino acids could be quantitated using an amino acid analyzer.
Lactate analysis. Acute conditions due to hypoxia, shock and impaired perfusion of tissue are often linked to lactate problems-lactic acid production. In addition, hereditary enzyme deficiencies leading to lacticacidemia have been reported in the pyruvate dehydrogenase system and the pyruvic carboxylase system, as well as in the key enzymes of the gluconeogenesis pathway. The last condition leads to both lactate acidemia and hypoglycemia.
The Lactate Analyzer (Roche Medical Electronics, Boston, Mass.) provides a rapid and accurate technique for analysis. It requires .1 ml of whole blood. The spread and accurate lactate determination could result in improved clinical handling of these critically ill infants. The measurement of lactate is based on the essentially irreversible oxidation of lactate to pyrvate in the presence of the enzyme cytochrome b2.
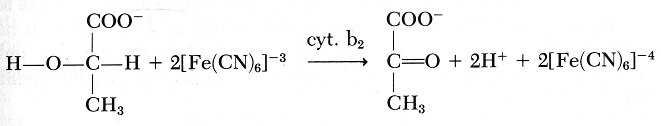
The hexacyanoferrate formed then is electrochemically oxidized: Pt
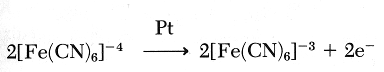
For each lactate oxidized, two electrons are collected on the platinum electrode. Hence, a current is measured. The reaction is first order kinetics and there is a linear relationship between the current developed and the lactate concentration of the sample.
BUN + Creatinine. Occasionally BUN and creatinine levels are estimated. High levels of BUN are secondary to dehydration from increased insensible water losses. Occasionally creatinine levels are elevated and the BUN/creatinine ratio is altered, which indicates renal failure due to hypoxia, drugs or primary renal disease.
The BUN Analyzer 2 (Beckman Instruments, Fullerton, California) determines blood urea nitrogen by means of the enzymatic conductivity rate method employing a Beckman conductivity electrode.
Precisely 3.0 ml of sample is manually pipetted into urease reagent in a cup containing an electrode that responds to changes in solution conductivity.
Solid-state electronic circuitry determines the rate of increase of conductivity, which is directly proportional to the concentration of urea in the sample. A digital display provides direct readout in milligrams of blood urea nitrogen per 100 ml.
SUMMARY
It is evident that a well-equipped laboratory is a pillar of an intensive care unit for critically ill infants. The provision of micromethods of blood glucose, bilirubin, electrolytes, blood gases and other specialized tests is an absolute necessity for frequent and close monitoring of the newborn's condition.[4] In addition, equipment duplication in such a laboratory is necessary to prevent delay in testing due to "down time."
The cost of such a laboratory set-up and round-the-clock trained personnel appears to be exorbitantly high, but it is nowhere near the expense for the care of an institutionalized handicapped individual if this set-up is not provided. Thus, it is beyond doubt that such a laboratory is not only important for the survival of a newborn but is also essential for the quality of the life saved.
REFERENCES
1. O'Brien D., Ibbott F. A.: Laboratory Manual of Pediatrics: Micro and Ultra Micro-biochemical Techniques. 3rd ed. Maryland: Harper & Row, 1964.
2. Wootan I. D. P.: Microanalysis in Medical Biochemistry. 5th ed. Edinburgh and England: Churchill-Livingstone, 1974.
3. Galjaard H.: Genetic metabolic disease: Early diagnosis and prenatal analysis. Amsterdam: Elsevier/North Holland, 1980.
4. Avery G. B.: Pathophysiology and management of the newborn. Philadelphia and Toronto: J. B. Lippincott Company, 1975