Retrolental Fibroplasia: A Modern Parable – Chapter 14
Chapter 14
The Future for Studies Involving American Children
Doubtless ye will say unto me this parable, Physician, heal thyself.
— Luke iv, 23
I have no prescription to cure the enigmatic social dysfunctions which have been outlined, and I have no intention of proposing palliatives to ease the pain. The problem-set of complex societal issues concerning research involving children in the United States does not lend itself to an inspirational solution. I do not agree with those who are seeking make-shift compromises to calm the fears of critics and allow everything to return to “normal.” Price has suggested that the present crisis of attitudes toward all research is an inevitable consequence of the characteristics of the growth curve of science as a whole, which has had a long life of purely exponential growth (Fig. 14-1). As this pattern of increase reaches the midpoint of the “natural” curve and enters a period of secession from the accustomed conditions of expansion, there is increasing concern over the problems of manpower, publication, and expenditure that demand solution by reorganization. If Price is correct, these are inescapable phenomena associated with the approach of the upper limit of scientific escalations. “If we expect to discourse in scientific style about science, and to plan accordingly,” he writes, “we shall have to call this approaching period [of the involvement of science with society] . . . Stable Saturation; if we have no such hopes, we must call it senility.”
There is a special need to seek stability in the relationships between perinatal research activities and the community-at-large. Anything which influences the quantity and quality of human reproduction has a magnified social impact (the term “environmental impact,” used in other contexts, is not entirely inappropriate here); as I said earlier, the effects extend well beyond the current generation. Health care development in perinatal medicine is, in essence, a political and social process; more than in any other field of medicine, there must be public participation in defining the goals of the medical effort. And there should be community evaluation of progress. An advisory panel to the Department of Health, Education and Welfare reviewed the question of whether or not policies (in the 1970s) to protect the rights of patients participating in government-supported health research were satisfactory. They concluded that it is the spirit in which our society undertakes the use of human beings for research which will determine the protection they receive. And the panelists urged much greater participation by society in the decisions which influence so many lives. Eisenberg has advised that there will be moral gain as well as health gain if we can create a community of shared responsibility for health research. An example of the kind of cooperative effort which is possible when there is unusually high motivation occurred in the polio vaccine trials conducted in the United States in 1954. Poliomyelitis had been one of the most dreaded diseases of childhood. But the fears were out of proportion to its total impact in children as compared, say, with automobile accidents. Nonetheless, it appeared in recurrent, seasonal waves, leaving many crippled children in their wake, and the threat of major epidemic outbreaks of the disorder was very frightening. American determination to combat this infectious condition was bolstered by the fact that President Franklin D. Roosevelt had been paralyzed as the result of polio acquired in early adult life. His former law partner, Basil O’Connor, led national campaigns to raise money for research to prevent or cure the disease. These fund-raising efforts supported investigations leading to Ender’s method of cultivating the polio virus in the chick embryo and to the development of the Salk vaccine. The hoped-for preventive measure was ready in 1954 to be field tested in trials involving school children. The object of the trials was to evaluate effectiveness of immunization in preventing the disorder and to detect any unforeseen complications of the vaccine. News reports explained these objectives and described the research methodology before the trials were begun. Health departments electing use of the experimental approach enrolled 401, 974 children; parental permission for participation was obtained and children received either Salk vaccine or ineffective salt solution as decided by random ordering (each assignment remained undisclosed until outcomes in all children were reported). Among 200,745 children in the vaccinated group, the rate of polio was 0.028 percent (of these 58 percent had paralytic complications, and there were no deaths); among 201,229 in the control group, polio developed in 0.071 percent (of these, paralytic complications occurred in 81 percent, and death occurred in 1.4 percent of those affected).
The polio vaccine trials demonstrated that when the American public perceives that it is threatened by a frightening “enemy,” there is whole hearted support and active participation, including the involvement of children as subjects in research trials. Although the vaccine-trials episode was a landmark in the history of communion between medical researchers and the public, paradoxically, the spectacular success of the effort has had some unfortunate after-effects. The polio experience raised expectations about medical omnipotence which were unreal, and it has led to enormous pressure for quick solutions to long-standing problems, notably in perinatal medicine. (The National Foundation-March of Dimes, organized for the efforts against polio, is now directing its attention to congenital malformations and other perinatal problems.) Regrettably, the uniqueness of the issues in problems of pregnancy and the newborn period have been inadequately stressed. Simply stated, the scientific evidence needed for decision-making must be of a higher order of completeness and credibility than that generated in the vaccine trials. Poliomyelitis, as I said, was a relatively uncommon disorder of childhood in the 1950s; it was responsible for 6 percent of all deaths in children; it crippled far fewer individuals, and the social consequences were not as far-reaching as the conditions which complicate pregnancy and delivery; importantly, there were no questions in polio survivors about the transmission of heritable defects to future generations, and there was no reason to suspect that the long-term cost to human society might wipe out the short-term gains. The stakes involved in decisions about interventions in perinatal matters are considerably higher than those which had to be considered in recommending a national policy for polio vaccination. Even in retrospect, the contrast between the extent of the successes achieved by the 1953-54 oxygen trial for RLF and the 1954 vaccine trials for polio does not convey fully the difference in magnitude and complexity of the two problems. Let me be understood. If the search for comprehension in matters concerning human procreation is to be more democratic, more thorough, and safer than in the past, it follows that innovations should be applied more slowly, and on a more limited scale than heretofore. These words may appear to be cheerless to those with a millenarian outlook who wish to see the solution to all of the outstanding problems in their lifetime. They may seem heartless to parents who wish to see a solution to a specific problem within their child’s lifetime. But I hope my formulation does not sound strange to those who value a search for the truth. For, I am not advising a reduction in the volume of basic biomedical research, and I strongly reject the notion that all laboratory research must be directed toward a specific clinical goal. (A review was made recently of the key observations which led to the ten most important advances in cardiovascular and pulmonary medicine and surgery in the last 30 years. Research efforts which had no foreseeable bearing on these clinical problems — the non-goal-directed investigations — paid off in terms of key discoveries almost twice as handsomely as other types of research and development combined.) I argue that the pace should slow at the threshold of bedside applications of proposals which arise from the findings in biomedical studies. My advice is exactly the opposite to the activist viewpoint which has been called the “Lyndon Johnson doctrine,” after his 1966 speech chiding the research community for its alleged failure to translate the findings of basic research speedily into practice. Proposed interventions, I contend, should be evaluated in cautious, graded steps, adhering to the same standard of scientific rigor used in preclinical research. But these applications should be sharply goal-directed. Overall progress in medicine is not measured by the rate of change, but the rate of advancement toward a goal. And, to repeat, the goal in perinatal interventions must not be defined exclusively by physicians. The limits of medical action have been clearly demarcated by Freidson: the conduct of medical practice or application of expertise is analytically distinct from expertise or knowledge itself. The distinction makes it clear that when decisions are fundamentally moral or evaluative rather than substantive, laymen have as much if not more to contribute to them than have experts. This assumption reflects the substance of equality in a free society, Freidson emphasized, equality not of ability, knowledge or means, but moral equality. Medicine must restore this balance if it is to reverse a disturbing trend: it is becoming an institution of social control.
The era of society’s involvement with science can begin when there is public realization that science values a search for understanding about the natural world. The search will become a source of American social values only when our society accepts the assumption that no belief will survive if it conflicts with factual evidence. The medical research community takes it for granted that it should pursue the truth, that the truth is still being pursued, and that the pursuit will go on always. The truth has not already been found. Bronowski pointed out that a society that believes that the truth is at hand, for example in politics or religion, simply imposes it; it is an authoritarian society. I believe as medicine moves away from its authoritarian past and becomes more scientific, it should abandon the “breakthrough” mentality (a “Eureka! We have found it!” warp which began with the discoveries of penicillin and polio vaccine). Physicians should become concerned with the continuing process of the search for understanding. There should be a heightened emphasis on the adequacy of the design of research. Medawar detected such movement in a review of the development of the scientific method over the past 300 years: inductive experiments to increase the store of factual information (of the type advised by Francis Bacon-through careful observation of a number of individual cases, a scientist hoped to “induce” a general statement about all cases) are being replaced by critical experiments carried out to test hypotheses or preconceived ideas. In the latter type of experiment, which seeks to discriminate among possibilities, problems of design are placed before those of validation. A similar trend in the use of the scientific method in clinical investigations is more difficult to perceive. For example, a recent review of the acceptance of the randomized clinical trial as the method of choice for evaluating clinical treatments indicated very slow progress. The experimental format rose from 0.3 percent of studies testing treatments in gastroenterology in 1964 to 1.7 percent in 1973. The authors concluded that randomized clinical trials would take over completely by the year 2010 provided the increase is exponential; in about 700 years if it is linear. An interview survey of 61 institutions conducting research in children (July 1974-June 1975) reported that random assignment methodology was used in about 1 out of 6 clinical studies (among 219 projects in which the investigators answered the question posed).
What can be done about improperly designed studies involving human subjects-studies that cannot possibly yield interpretable results? I have argued throughout this book that improvement in scientific rigor inevitably results in improved protection for the participants in medical studies. And a remedy suggests itself from the historical trend in the development of the scientific method: an increased preoccupation with methodology. Editors and review committees of medical journals have it within their power to prescribe a cure: a change in the primary criterion for acceptance of reports describing bedside studies, from an emphasis on results to an insistence on proper design. Walster and Cleary found a similar problem with studies in the social sciences and they proposed a new editorial policy for articles which make inferential statistical analyses of results. The cardinal rule in experimental design, they argued, is that any decision regarding the treatment of data must be made prior to the inspection of the data. If this rule is extended to publication decisions, it follows that when an article is submitted for review, the data and the results should be withheld. This would guarantee that the decision to publish, or not to publish, would be unrelated to the outcome of the research. The editorial decision would be based upon such factors as the adequacy of the design, and the relevance of the research to theoretical and topical issues. Radical as this proposal sounds at first hearing, it is quite logical and entirely practical. I believe the plan deserves serious consideration.
The challenge for the future in clinical studies involving babies is to develop acceptable processes for changing the ordered pattern of response to new proposals: formal evaluation should be undertaken before, not after, wide use of new treatments and procedures. However, there should be improvements in the approach which relate to scientific and to democratic principles (and the two are not antithetical, they are complementary). I believe small-scale explorations of various approaches (experiments in the procedure of bedside experimentation) are in order, and I wish to make several proposals which might be considered in a search for improvements. These are related to public involvement and concerns in clinical studies, the categorization of risks and benefits, a schema for informed surveillance in clinical trials, and, finally, a Popperian approach to experimental tactics.
Clinical studies have been conducted more frequently among impoverished minorities than among the privileged American classes. The poor became subjects on whom studies were done because of their convenience (researchers were located in the teaching hospitals used by poor patients) and because of gross insensitivity to the unfairness of the practice. To the extent that the disadvantaged young were not representative of all American children, the sampling bias in the past was an example of poor science and poor democracy. The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research (established by an act of Congress in 1974) has recently advised that children who participate in research projects should be selected so that the burdens of participation are distributed equitably among the segments of our society. But how is this to be accomplished? Volunteering thwarts the basic principle of random sampling (the latter is used to assure equality of representation), and there are gross social inequities. For example, Jonas advised that the general criteria for participants in clinical experimentation should include the very qualities least likely to be found among those on the lowest rungs of the social ladder: a maximum of identification, understanding and spontaneity. Researchers should look for subjects among the most highly motivated, the most highly educated, and the least “captive” members of the community, he advised. These qualities describe physicians and their families, but it has been my long and consistent experience that they are unwilling to allow their own children and grandchildren to participate in research projects. It seems to me that conscription-by-lot, however unpopular, is an honorable solution to the dilemma posed by the Commission’s recommendation. Jonas discussed this approach and rejected it on the grounds that it was an unacceptable philosophy. Indeed, he viewed it as threatening and utopian, an approach from which we should recoil. I appreciate his horror, but I suggest that it stems from the popular (pejorative) opinion that the subjects in human investigations must undertake unwarranted risks. If, on the other hand, systematic bedside research is viewed realistically, as the only practical means for protecting individuals from unknown risks in medicine, perhaps the rejection may not be quite so final. Democratic societies have used a draft to choose young men to defend the populace against real and imagined enemies, and medical warfare is no less real. Obviously, extensive educational efforts will be required to convince Americans that unassessed changes in care-taking practices may produce effects that are as devastating as the consequences of enemy action in war. But a glance at the numbers of infants who died or who were left blind, brain damaged or malformed in modern treatment disasters should be sobering: the totals are greater than in the polio epidemics-they rival wartime statistics!
Similar heroic and more specific efforts will be required to convince Americans that the under-utilized randomized clinical trial is the most powerful defensive weapon against the backfire of modern arms in medical arsenals. Although I argue that the educational efforts should be undertaken, I know that it will not be easy. Scientifically rigorous clinical study is slow and doctors in the front-lines of medical practice are under enormous pressure to “do something.” For example, the early observations that supplemental oxygen appeared to be useful in the management of premature infants made it very difficult for experienced physicians and nurses to consider testing effects which they found to be self-evident. Conscientious doctors, confronted with the problems of pneumonia in newborn infants (complicating the influenza epidemics in the 1950s), felt they could not wait for the results of a formal trial of the effectiveness and safety of chloramphenicol. But if these undisciplined approaches are to change, public pressure on physicians must change. A call for action should take into account that the modern doctor is more like a weapons specialist manning the push-button console of a missile launcher than a romantic warrior armed with a sword. Modern conditions call for cautious tactics: limited forays and planned battles against ignorance concerning medical matters. Simple concern for the public interest dictates that we can no longer ignore Claude Bernard’s maxim: science teaches us to doubt and, in ignorance, to refrain.
The classification of clinical investigations into two arbitrary categories (“therapeutic” and “nontherapeutic”, see p 117) is an illusory dichotomy which encourages subterfuge. Guidelines for the conduct of studies prepared by the American Academy of Pediatrics in 1977 attempt to distinguish between the indistinct classes; but the set of recommendations proposed by the National Commission abandons the distinction. I believe that in all studies, regardless of the purported intent, the relationships between risks and benefits should be considered on a continuous scale. At one end of the range there are procedures with virtually no benefit which should be balanced by practically no risk; at the other extreme the risks are so great that they outweigh any conceivable benefit. In addition, I suggest that categories of risks and benefits should be weighted differentially: heaviest weights assigned to the category of persons (or biologic unit in the case of the fetus or suckling, as I will discuss in a moment), a somewhat lighter weight to family, still lighter to subculture, community and so on (Fig. 14-2). 1 do not mean to imply that there can be a formula for computing the worth of research studies in perinatal medicine. But I do suggest that in our plural society, dominant-culture definitions of “risk” and “benefit” should not be applied across-the-board. For example, what is the risk-to-benefit ratio of a proposal to investigate the pharmacologic control of labor and delivery with the drug oxytocin as compared with standard obstetric practice? The purported benefit of this hormone is efficient induction and augmentation of uterine contractions during labor, and the possible risk is a small increase in jaundice among newborn infants leading to a small possibility that treatment of the jaundice will be required by exchange blood transfusion. The risk-to-benefit ratio is very much higher for Jehovah’s Witnesses (Chapter 12), I argue, than for others who define the risk of blood transfusion in Earth-bound terms. The grounds for exclusion of Jehovah’s Witnesses from such a trial are essentially the same as those for excusing conscientious objectors in conventional warfare. For the question of who should be called upon to participate in clinical studies can only be made “right,” Jonas advised, by such authentic identification with the “cause” of the study that it is the subject’s as well as the researcher’s “cause”.
In the hypothetical example which I have chosen, the biologic status of the mother and her about-to-be-delivered infant is straightforward: they are a single biologic entity called the feto-maternal unit. Although this entity changes in physical and physiological detail at the time of birth, the newborn infant continues in an intimate and essential relationship with the mother. The relationship persists for a relatively long period of time. Bostock suggested that man lies somewhere between those mammals whose young walk at birth and the marsupials (like the kangaroo whose young migrate, at a very early stage of fetal life, out of the uterus into a pouch on the mother’s abdomen). He argued a thesis which he called “exterior gestation” (comparable to the marsupials): human fetal existence does not end with emergence into the air-breathing world, but with locomotion. To Bostock, human gestation is about 18 months long: 9 months inside and 9 months outside of the uterus. I will not defend this fanciful proposal (although there is a considerable body of evidence which shores up this general idea), but I quote it to make the point that the closeness of this relationship in man should not be underestimated when considering the issues involved in conducting studies during the perinatal period. The archaic term “suckling infants” (which I wish to revive) serves to emphasize the functional facts: mother and child form a symbiotic union-the mother-infant dyad.
In sharp contrast to this biologic view which stresses the oneness of the pair, theologic and legal views emphasize the separateness. Ramsey and Bartholome have written movingly about the fetus and the suckling infant as “persons.” And, the language of “rights” has been used to attempt to define their interests as research subjects, particularly in the matter of “informed consent” (who can give permission for the helpless fetus or suckling?). Needless to say, there is very little agreement here. Hauerwas examined some of the arguments and concluded that the issues are well-nigh insoluble in our cultural situation. I agree. Moreover, I believe it has been the arrogant intrusion of religious theories into these secular matters which has served to tangle the issues into a hopeless knot. I do not think the best interests of American families will be served by investing more time and endless arguments in the hope of materializing the mirage of “informed consent.” Eisenberg has correctly pointed out that the very justification for a randomized trial is that there is insufficient information to permit a rational, that is, informed choice. In a free society, we reserve the right for any citizen to opt out. But when we respect the privilege to be guided by superstition, astrology, or simple orneriness let us drop the adjective “informed” and speak only of “consent”, he concluded. To make the rout complete, the National Commission has abandoned the use of the word consent. In its recommendations, the commission has advised that “permission” of parents or guardians be solicited, to distinguish what a person may do autonomously (consent) from what one may do on behalf of another (grant permission). Additionally, it has been advised that a parent or guardian should be present as much as possible during the conduct of studies in infants. The last recommendation, active participation of parents, deserves special attention. I suspect, for example, that much of the anger and bitterness felt by parents in the years after the RLF incident would have been ameliorated if they had been encouraged to “be present as much as possible” when their infants were in the nursery. If parents had shared the anxieties of nurses and physicians concerning infants in both oxygen-management groups in the oxygen trials, there would have been an opportunity for the development of mutual understanding. It is even conceivable to me that the final results of the Cooperative Study in 1954 might have been questioned more by parents than physicians, if they had been active participants.
I propose a plan, informed surveillance, which might be used to explore the possibilities of expanding the roles of the principals involved in clinical trials (Fig. 14-3). In addition to the inclusion of parents, the plan places special emphasis on an active role for front-line practicing physicians, for reasons that I will discuss in a moment. We developed a version of this schema for safeguarding studies in premature infants at Babies Hospital in 1953. Our approach was stimulated by a point of view expressed by Guttentag in that year. He noted that the patient-physician relationship may be viewed from two aspects: in one, the physician is an objective, critical investigator; in the other, he is a responsive warm friend who is attentive to the cry for help from his fellow human beings in distress. In present-day highly technical investigation, Guttentag advised that the two facets of the patient-physician relationship be represented by two persons. The “physician-friend” in this division of responsibilities acts to defend the patient’s rights and personal welfare when a procedure is proposed by the “physician-investigator.” In the model which I propose, there is interaction of three parties (after institutional review and approval of a formal protocol of procedures to be used in a proposed study): (1), the physician-investigator, who is charged with the responsibility of informing the personal physician and answering questions on every detail of the study; (2), the personal physician, who is charged with the responsibility of attempting to inform parents about the details of the study and to reply to their questions which may arise before and at all stages of the investigation; and (3), parents, who are asked to give tentative permission for enrollment in the investigation only by indicating that they have no objection to proceeding. It has been pointed out that an explanation and an offer of choice simply are not enough to obtain informed consent (to use the abandoned terms for a moment). The quality of consent is not the same when the social distance between doctor and parents is great, as it is when they are more nearly social equals. The veto options of parents and of personal physician should be retained by them at all stages of the investigation. They should not be made to feel any obligation to adhere to the agreement made at the time of enrollment in a clinical trial; “second thoughts” should be respected without coercion.
If veto options are exercised so frequently that a trial is “ruined,” the significance of this turn of events should not be overlooked. From a community-oriented perspective, the rates of noncompliance and defection by parents and personal physician are basic pieces of information. Since the entire raison d’ etre for human experiments lies in the potential for projecting results to the community-at-large clinical trials must be designed to generate useful information bearing on this fundamental objective. If parents and personal physicians are unable to identify with the goals of the trial and do not perceive themselves as active participants, there is little reason to expect that an outcome of interest to the investigator will be of any public interest.
As I have already indicated, special efforts should be made to enlist the aid and to stimulate the interest of practicing physicians in formal clinical studies. For, it is the practitioner who must translate the results of these efforts into everyday practice. Chalmers has documented some examples of the disturbing effect when physicians feel alienated from research efforts. He examined the extent to which the practice of medicine is a reflection of controlled clinical trials. There was a clear-cut dichotomy between the “usual practice of the community” and the hard-won scientific data. Physicians seemed to be paying no attention to the reports of carefully designed studies. This happened after the 1954 study of RLF; physicians disregarded the results of the massive trial and administered oxygen to premature infants in concentrations below 40 percent in the belief that this was safe (Chapter 7). During the same period another disturbing incident occurred in which pregnant women received DES (Diethylstilbestrol). In the early 1950s this hormone was used to treat early complications of pregnancy, particularly in the hope of preventing miscarriage in women with a past record of habitual fetal loss. At the time of its use, there was no known toxicity. Enthusiastic endorsements were based on the results of seven uncontrolled observational studies, but six investigations found no beneficial effect. All six of the studies which gave negative results used controlled-trial methodology and three of these trials were double-blind (neither physician nor patient knew, in each instance, whether hormone or inactive placebo had been given, and the identification of patients as “treated” or “control” was not disclosed until after the outcomes were recorded). As Chalmers has pointed out, these findings should have been impressive to practicing physicians. The evidence that the estrogenic drug had no effect in preventing miscarriage in any group of patients was quite substantial. Six of seven textbooks of obstetrics came to this conclusion in the 1960s. Despite this, during the period between 1960 and 1970 an average of ca. 100,000 prescriptions for DES per year were written for women who were pregnant. And there was evidence of geographic differences: (1) use of the drug for prenatal care was more “fashionable” in the East and Midwest than in the South and West (3.67 and 2.93 vs 1.94 and 1.68 DES prescriptions per 100 live births), and (2) use of the hormone varied considerably between hospitals located in different areas of the United States (from 1.5 percent of pregnancies in a Boston hospital to zero among 3460 pregnancies in a Baltimore hospital). This was years after evidence from the controlled studies indicated that the treatment had no demonstrable beneficial effect. Beginning in 1972, it was observed that cancer of the vagina was developing in some young women whose mothers had received the hormone while pregnant. The total dimensions of the DES disaster have not yet been determined.
Undoubtedly, many proposals will be made to find a way through the maze of difficulties which complicate use of the experimental method in clinical studies involving the fetus and suckling infant. The National Commission’s report serves a useful, but limited, function: it indicates where the wrong turns are located. However, I find it hard to believe there will be anything more than token movement until parents, practicing physicians, ethicists, lawyers and legislators insist on the use of scientifically rigorous methodology because of the inherent safety of the approach. Additionally, general acceptance of new treatments in the future may involve governments and voters who demand a slower but better-coordinated advance in this field of activities; a pace that does justice to the processes, functions, and purposes of life. H. Mahler, Director-General of the World Health Organization, has said that objective assessment must go beyond technical considerations. Communities have a right to make value judgments concerning new developments. Mahler advised that critical evaluation of new interventions must entail not only controlled clinical trials, but an additional step: controlled community trials on ways of delivering fully assessed technologies to specific communities. The Director-General was referring to the problems which have resulted from the unevaluated use of complex medical technology in underdeveloped countries. But his cautions should be considered in the developed countries as well. For instance, the criticism of the Oxford consumer group (p 110) suggests that even after perinatal intensive care technology has been fully evaluated from a medical point of view, it should not be imposed on a community without a planned trial to evaluate the full social effects of this intervention.
Finally, I propose exploration of a change in experimental strategies based on a different approach to the theory of scientific knowledge: a shift from the validation-of-hypotheses tack to Popper’s “refutability” formulation. He observed that science is not a system of certain or well-established statements; nor is it a system which advances toward a state of finality. Since it can never claim to have attained the truth, the method of research should not defend conjectures in order to prove how right we are. And, since a single definitive refutation stands out against countless confirmations, Popper advised that we try to overthrow suppositions, using all the logical, mathematical, and technical weapons available. (No matter how many thousands of times that loud noise has been shown to “cure” a solar eclipse, a single test consisting of a few minutes of silence is enough to overturn the theory.) Popper holds that all theories are tentative, never certain: a scientific “truth” is merely a statement which has thus far resisted refutation. Moreover, the requirement that statements should be capable of being proven erroneous sets them apart from metaphysical statements which are not refutable by any conceivable event (Galen’s claim for his failure-proof remedy is a good example of a metaphysical assertion in medicine, Chapter 13).
Popper’s thesis that test-by-refutation has a privileged place (over that of test-by-verification) is not acceptable to some students of the philosophy of science; but I do not wish to become involved in the controversy. The point I wish to make is that his refutation approach has some important practical advantages as a theoretical base for clinical studies; for, in bedside medicine the temporality and provisional nature of “truth” is taken for granted. Experimental designs to place proposals at maximum risk of invalidation require considerably more planning and ingenuity than are needed to mount corroborative studies. Most importantly, the aim of a severe test is not necessarily the total destruction of each theory, but to obtain information about the limits of its applicability (or safety). For example, at the conclusion of the 1954 Cooperative Study of RLF this statement appeared in the final report: “Limiting the duration [of stay] in oxygen to that deemed necessary to meet frank clinical emergency was shown to be without effect on the survival rate of the premature infant.” From the Popperian point of view which I propose, one is obliged to ask: What are the limits of applicability of this assertion?, and, importantly, Was the 1954 test of the long-held fears of oxygen curtailment a vigorous test? The answer to the second question is, No. The mortality risk among the infants in the study (all were enrolled after reaching the age of 48 hours) was relatively low when compared with the risk in the first two days of life. The curtailment-of-oxygen-is-safe theory was not tested under conditions where it would be placed in maximum jeopardy. As a result, the 1954 declaration of “no significant difference” in mortality (setting aside the semantic problems which surround the phrase, see chapter notes) should be regarded as very provisional, indeed. Popper notes the paradox that the more a theory says, then the more it excludes or forbids, and the greater the opportunities for falsifying it. This relationship between greater content and higher falsifiability is seen in these two assertions:
- Curtailment of oxygen for premature infants is safe.
- Curtailment of oxygen for premature infants under 48 hours of age is safe.
The content of the second statement is greater than the first: it provides more information. But the second excludes more than the first: it prohibits an acute protective effect confined to the first two days of life. And it has more opportunity to be falsified. This is the essence of Popper’s thesis: our knowledge grows by the process of replacing any given assertion with a more risky theory — one less likely to hold up to experiments or tests.
Obviously, a critical test of the effects of oxygen restriction in the first 48 hours of life would have required considerable thought, planning, and care if the disputed issues were to be examined in a responsible manner. And it would have been a very difficult trial to carry out. But the results would have provided some objective evidence concerning a problem which has plagued physicians for almost a quarter of a century. And it goes without saying that the well-being of countless infants would have been protected by the critical approach which seeks to narrow the area of uncertainty. In this regard, the Popperian aim is just the opposite of that in evangelism which seeks to convert and to generalize without limit.
It is relatively easy to make any theory appear credible if we concentrate on collecting confirmatory observations from the world of experience. For example, the initial observations on the success of ACTH, oxygen curtailment, detergent-mist, half-skimmed cow’s-milk mixtures, and DES were all supported by repeated confirmatory observations. Only the attempts to put each of these clusters of favorable experiences (like the runs of consecutive “heads” in coin-tossing) to a formal test, a test which risked the possibility that the initial good results would not be confirmed, succeeded eventually in narrowing the areas of uncertainty about the proposals. Perhaps the lessons from the past and the implication of Popper’s logic will change the emphasis from validation to limits of application in future studies involving children. I sincerely hope so.
Scio (I know), the presumptuous slogan of medicine, should, at long last, be replaced by the modest Quaero (I seek). This was fully appreciated by Claude Bernard. In reference to the tentative nature of results obtained after the most painstaking methods to arrive at the “truth,” he said,
There are only partial and provisional truths which are necessary to us as steps on which we rest so as to go on with investigations.

A. General form of the logistic growth curve of science in the United States described by Price. The steady state of exponential growth is followed by a decline to linear growth and finally an exponential rate of deceleration. He estimated that only about 30 years must elapse (from the “present state” in 1959) between the period when some few percent of difficulty is felt and the time when that trouble has become so acute that it cannot possibly be satisfied.
B. The “growth curve” of medical knowledge estimated by Durack from the weight of the annual volumes of Index Medicus (the National Library of Medicine’s bibliography of the literature of biomedicine).
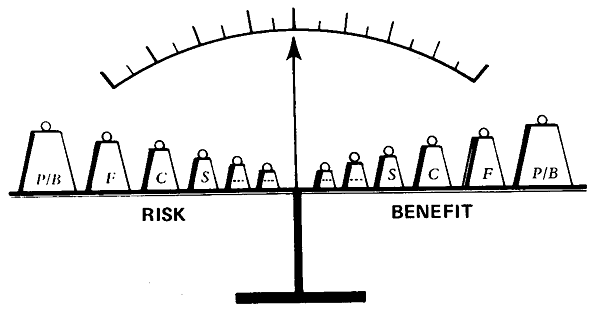
Risks and benefits are appraisals; differential weightings (person/ biologic unit, family, community, subculture . . .) are supplied by different social groups.
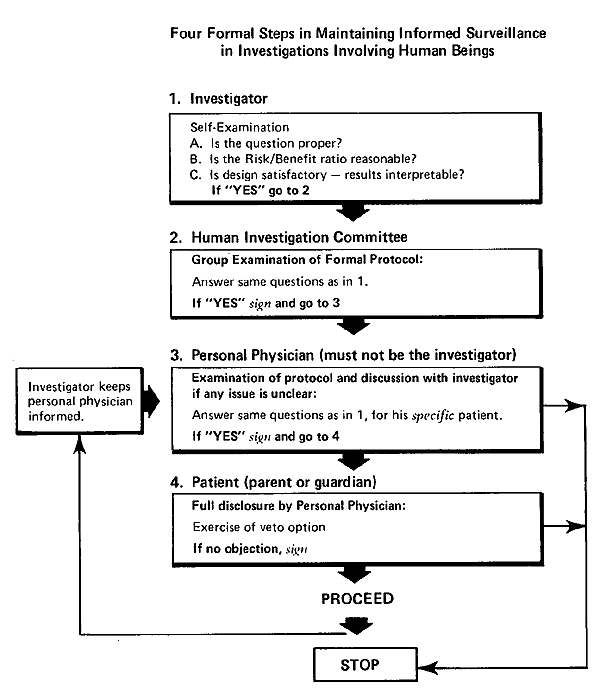
Flow diagram of a model for informed surveillance in clinical studies.
Last Updated on 02/28/24