Retrolental Fibroplasia: A Modern Parable – Chapter 5
Chapter 5
The Eye and Oxygen
In the early 1950s there was a vague feeling that the key to solving the mystery of RLF might lie in improved understanding of the unusual way in which the retinal layer of the eye receives its blood supply during fetal development. Ida Mann, in 1928, described the embryonic development of the human eye and developed the concept that the retinal vessels originate by budding from the base of the fetal blood vessel of the eye (hyaloid artery; see chapter notes). Twenty years later, 1. C. Michaelson made observations concerning the fine details of development of the blood vessels and capillaries of the retinal layer. He used a technique which consisted of injecting India ink into the arterial system to fill and blacken the smallest vessels of the fetal eye, removing the globe for dissection, then teasing out the retinal layer to make a flat preparation which he mounted on a glass slide. This allowed him to map the extent of development of patent vessels at different stages of fetal development in a number of species from eels to man.
According to the view established by these observations (see chapter notes for later views), the human retina has no blood supply of its own until the third month of fetal life. This was interpreted to indicate that retinal tissue receives adequate amounts of oxygen and nutrient from the vessels of the choroid layer of the eye which lies just beneath the retina. Michaelson suggested that as the retina develops and becomes thicker at the third to fourth month of gestation, the nutritional needs can no longer be met by the nearby choroid. At the fourth month, a small swelling was seen on the trunk of the hyaloid artery as it passes into the eyeball, at the point where the optic nerve joins the globe. This was considered to be the site of origin of the newly emerging retinal arteries, which appeared to grow outward, reaching the edge of the retina relatively late in the development of the eye.
Michaelson suggested that retinal capillaries sprout from the new vessels which have grown out from the region of the optic nerve. He pointed out that when the veins and arteries are close to one another, capillary growth appears to take place from the side of the vein away from the artery (Fig. 5-1). A capillary network of varying density is seen in the space between the arteries and veins, becoming more abundant as the veins are approached. There is a zone which is free of capillaries around the arteries. Michaelson concluded
… there is present in the developing retina a factor which affects the budding of new vessels … it is present in a gradient of concentration such that it differs in arterial and venous neighborhoods …
F. W. Campbell, intrigued by these observations, set out to define Michaelson’s “factor.” He demonstrated that the capillary-free zones around the arteries in newborn rats (development of the retinal vessel system is similar to that in man) were significantly narrower when the animals were placed in a low-oxygen chamber than in control rats of the same age left in room air. Campbell postulated that an oxygen debt in the developing retina, which can no longer be met by the underlying choroidal vessels, serves as the stimulus for the normal fetal outgrowth of the new system of vessels from the region of the optic nerve. According to this hypothesis, the new blood channels form in response to the growing oxygen needs of the maturing retina.
The relevance of these provocative ideas to RLF went virtually unnoticed for several years. The effects of exposure to low (subatmospheric) oxygen on the immature eye of mice, and the effects of intermittent high oxygen on newborn mice, were reported in 1952. However, abnormal changes in the eye found in these studies were not generally accepted as those of typical human RLF. Young kittens given excessive amounts of salt and water developed swelling of the retinal layer of the eye, but, again, the microscopic appearance of the eyes was not the same as that seen in RLF.
As the nursery controversy concerning the role of oxygen in RLF raged on in 1953, Norman Ashton, a pathologist at the Institute of Ophthalmology, University of London, became interested in the puzzle. He came to the problem with knowledge that the newborn kitten had incomplete blood vessel formation in the retina, roughly comparable to the human premature infant at about 7 months of gestation, and he was armed with the elegant India ink injection technique for retinal study. He borrowed a bacteriology incubator from a pediatrician who had been planning to do some oxygen studies of RLF and converted it to a gas chamber to administer high concentrations of oxygen. With his co-workers, Basil Ward and Geoffrey Serpell, Ashton put a mother cat and three kittens in the chamber and measured the oxygen concentration in the device at half-hour intervals day and night. The first experiment showed that after four days in continuous high oxygen (60-70 percent concentration), the outgrowing retinal vessels were completely withered (Fig. 5-2). The London team went on to demonstrate that constriction of immature vessels was the primary effect of high oxygen. If maintained for a long enough period, obliteration of the entire developing blood vessel network of the retina took place. Regrowth of blood vessels after return to air took place in a wild disorganized fashion with budding of new capillaries into the vitreous portion of the eye in front of the retina (Fig. 5-2). This was consistent with the early blood vessel changes in RLF noted in the human by Reese and Blodi in 1951 and amply confirmed by others.
Ashton regarded RLF as no more than a “violent activation” of the normal process of retinal vessel development, precipitated by an exaggerated lack of oxygen in the deep retinal layers, as described earlier by Campbell. Ironically and paradoxically, it was exposure to high oxygen concentration, with resultant obliteration of developing retinal blood vessels, which appeared to cause the ultimate oxygen deficiency in the deep retinal layer of the eye. These results seemed bizarre: breathing high concentrations of oxygen appeared to render one tissue of the body oxygen-impoverished! On the other hand, the findings also provided a basis for some reconciliation with the views of those who considered overall oxygenlack to be the cause of RLF. Although Ashton’s experiments indicated that low oxygen, consistent with the survival of his animals, usually was not sufficient to give rise to proliferation of retinal blood vessels, he suggested the possiblity that severe oxygen-lack might, on occasion, produce the disease directly.
In 1953, Patz and his co-workers conducted extensive studies of high oxygen exposure in several species of newborn animals, beginning with opossum and rats and later with mice, kittens, and puppies, with the same results as those reported by Ashton. Although these elegant animal studies were impressive, there was one substantial problem in extending the findings to human RLF; this was voiced by Ashton:
The findings exactly parallel the early stages of the human disease. The difference in subsequent course is dependent upon the development of retinal detachment which did not occur in the kitten experiments.
In other words, the blood vessel changes of RLF in newborn kittens and other experimental animals did not go on to scarring (cicatricial) RLF. The experimental animals did not become blind!
By the beginning of 1953, the next step seemed clear to me and to many of my peers who were responsible for the day-to-day care of premature infants. We were worried about the harmful consequences of oxygen restriction and felt that further study in babies was the only way to resolve the substantial doubt concerning the exact role of oxygen in human RLF.

Appearance of the injected retina of a human fetus, from Michaelson’s studies. He considered that capillary growth occurred from the veins forming a capillary-free space around the arteries. Note that the small veins which cross the artery do not branch into capillaries until they have passed the arterial vessel by a certain distance. (Michaelson’s concept has been modified by later findings; see chapter notes.)
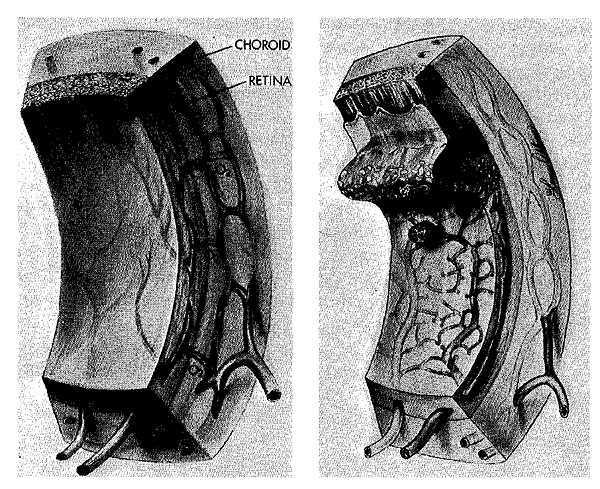
Effect of oxygen in RLF. Left: Initially the retinal vessels are constricted, then obliterated, and peripheral outgrowth is suppressed. Arrows indicate increased diffusion from high oxygen tension in the neighboring choroidal blood vessels, which are not appreciably constricted. Right: Later, the withered blood vessels of the retina regrow in a disorganized fashion; proliferating capillaries erupt through the retinal surface into the vitreous humor. Only the incompletely developed retina is susceptible to this effect of hyperoxia. Once blood vessel development is complete, supplemental oxygen causes a decrease in the caliber of the mature retinal vessels, but pronounced vasoconstriction and obliteration do not take place (see chapter notes, Fig. 9-1, and notes for Chapter 9).
Last Updated on 02/28/24